Novel Curcumin-Diethyl Fumarate Hybrid as a Dualistic GSK-3β Inhibitor/Nrf2 Inducer for the Treatment of Parkinson's Disease
- PMID: 32663009
- PMCID: PMC8009478
- DOI: 10.1021/acschemneuro.0c00363
Novel Curcumin-Diethyl Fumarate Hybrid as a Dualistic GSK-3β Inhibitor/Nrf2 Inducer for the Treatment of Parkinson's Disease
Abstract
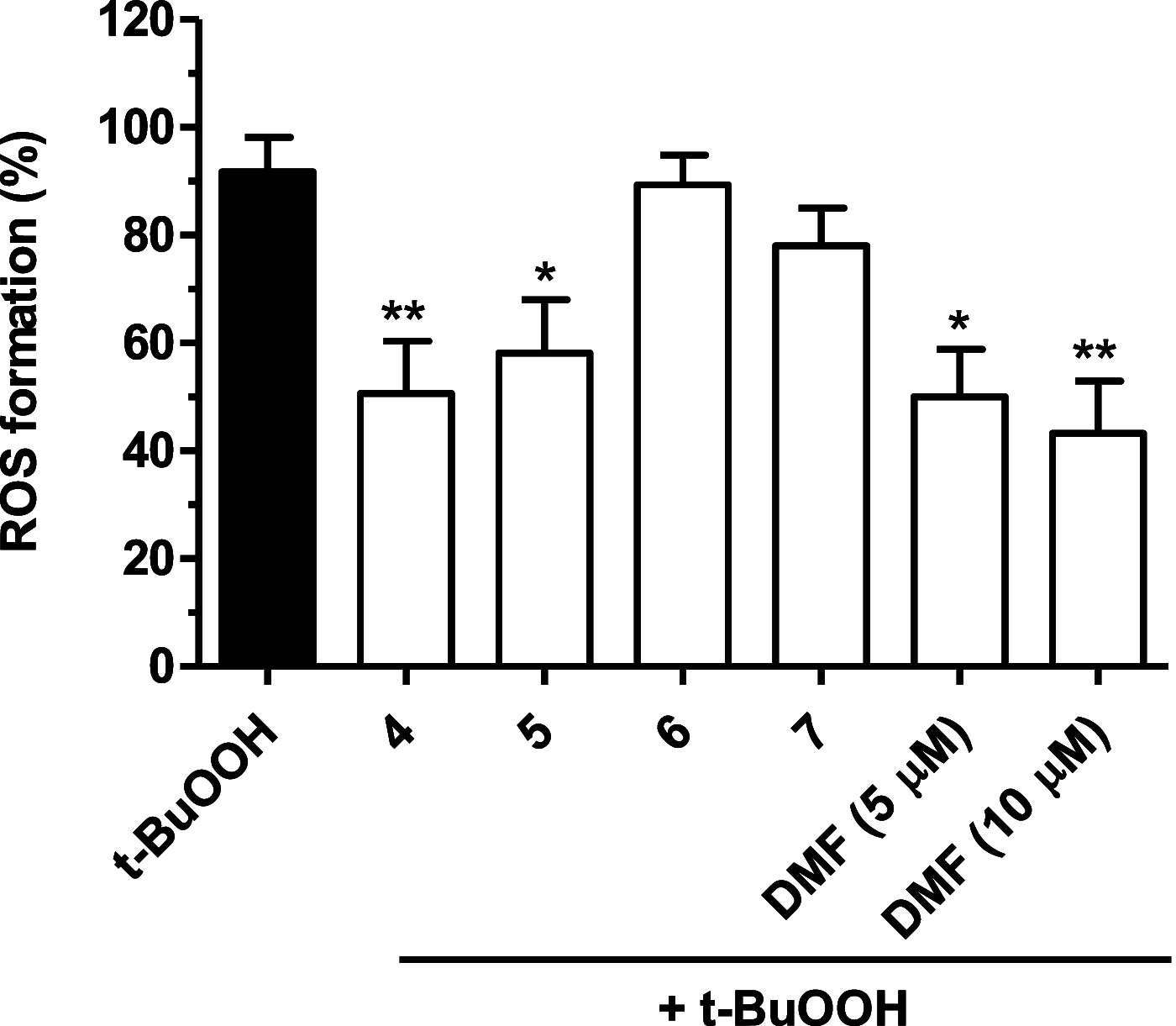
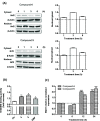
Common copathogenic factors, including oxidative stress and neuroinflammation, are found to play a vital role in the development of neurodegenerative disorders, including Alzheimer's disease (AD) and Parkinson's disease (PD). Nowadays, owing to the multifactorial character of the diseases, no effective therapies are available, thus underlying the need for new strategies. Overexpression of the enzyme GSK-3β and downregulation of the Nrf2/ARE pathway are responsible for a decrease in antioxidant defense effects. These pieces of evidence underline the usefulness of dual GSK-3β inhibitors/Nrf2 inducers. In this regard, to design a dual modulator, the structures of a curcumin-based analogue, as GSK-3β inhibitor, and a diethyl fumarate fragment, as Nrf2 inducer, were combined. Among the hybrids, 5 and 6 proved to effectively inhibit GSK-3β, while 4 and 5 showed a marked ability to activate Nrf2 together to increase the neuronal resistance to oxidative stress. These last pieces of evidence translated into specific neuroprotective effects of 4 and 5 against PD pathological events including neurotoxicity elicited by α-synuclein aggregates and 6-hydroxydopamine. Hybrid 5 also showed neuroprotective effects in a C. elegans model of PD where the activation of GSK-3β is intimately involved in Nrf2 regulation. In summary, 5 emerged as an interesting multitarget derivative, valuable to be exploited in a multitarget PD perspective.
Keywords: Curcumin analogues; Diethyl fumarate; Glycogen synthase kinase-3β; Neurodegeneration; Nuclear factor-erythroid related factor 2; Oxidative stress; Parkinson’s disease.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Durrenberger P. F.; Fernando F. S.; Kashefi S. N.; Bonnert T. P.; Seilhean D.; Nait-Oumesmar B.; Schmitt A.; Gebicke-Haerter P. J.; Falkai P.; Grunblatt E.; Palkovits M.; Arzberger T.; Kretzschmar H.; Dexter D. T.; Reynolds R. (2015) Common mechanisms in neurodegeneration and neuroinflammation: a BrainNet Europe gene expression microarray study. J. Neural Transm (Vienna). 122, 1055–1068. 10.1007/s00702-014-1293-0. - DOI - PubMed
- Gan L.; Cookson M. R.; Petrucelli L.; La Spada A. R. (2018) Converging pathways in neurodegeneration, from genetics to mechanisms. Nat. Neurosci. 21, 1300–1309. 10.1038/s41593-018-0237-7. - DOI - PMC - PubMed
-
- Cheng J.; North B. J.; Zhang T.; Dai X.; Tao K.; Guo J.; Wei W. (2018) The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease. Aging Cell 17, e12801 10.1111/acel.12801. - DOI - PMC - PubMed
- Lehtonen S.; Sonninen T. M.; Wojciechowski S.; Goldsteins G.; Koistinaho J. (2019) Dysfunction of Cellular Proteostasis in Parkinson’s Disease. Front. Neurosci. 13, 457. 10.3389/fnins.2019.00457. - DOI - PMC - PubMed

