Ten-Year Results of FAST: A Randomized Controlled Trial of 5-Fraction Whole-Breast Radiotherapy for Early Breast Cancer
- PMID: 32663119
- PMCID: PMC7526720
- DOI: 10.1200/JCO.19.02750
Ten-Year Results of FAST: A Randomized Controlled Trial of 5-Fraction Whole-Breast Radiotherapy for Early Breast Cancer
Abstract
Purpose: Previous studies of hypofractionated adjuvant whole-breast radiotherapy for early breast cancer established a 15- or 16-fraction (fr) regimen as standard. The FAST Trial (CRUKE/04/015) evaluated normal tissue effects (NTE) and disease outcomes after 5-fr regimens. Ten-year results are presented.
Methods: Women ≥ 50 years of age with low-risk invasive breast carcinoma (pT1-2 pN0) were randomly assigned to 50 Gy/25 fr (5 weeks) or 30 or 28.5 Gy in 5 once-weekly fr of 6.0 or 5.7 Gy. The primary end point was change in photographic breast appearance at 2 and 5 years; secondary end points were physician assessments of NTE and local tumor control. Odds ratios (ORs) from longitudinal analyses compared regimens.
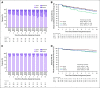
Results: A total of 915 women were recruited from 18 UK centers (2004-2007). Five-year photographs were available for 615/862 (71%) eligible patients. ORs for change in photographic breast appearance were 1.64 (95% CI, 1.08 to 2.49; P = .019) for 30 Gy and 1.10 (95% CI, 0.70 to 1.71; P = .686) for 28.5 Gy versus 50 Gy. α/β estimate for photographic end point was 2.7 Gy (95% CI, 1.5 to 3.9 Gy), giving a 5-fr schedule of 28 Gy (95% CI, 26 to 30 Gy) estimated to be isoeffective with 50 Gy/25 fr. ORs for any moderate/marked physician-assessed breast NTE (shrinkage, induration, telangiectasia, edema) were 2.12 (95% CI, 1.55 to 2.89; P < .001) for 30 Gy and 1.22 (95% CI, 0.87 to 1.72; P = .248) for 28.5 Gy versus 50 Gy. With 9.9 years median follow-up, 11 ipsilateral breast cancer events (50 Gy: 3; 30 Gy: 4; 28.5 Gy: 4) and 96 deaths (50 Gy: 30; 30 Gy: 33; 28.5 Gy: 33) have occurred.
Conclusion: At 10 years, there was no significant difference in NTE rates after 28.5 Gy/5 fr compared with 50 Gy/25 fr, but NTE were higher after 30 Gy/5 fr. Results confirm the published 3-year findings that a once-weekly 5-fr schedule of whole-breast radiotherapy can be identified that appears to be radiobiologically comparable for NTE to a conventionally fractionated regimen.
Trial registration: ClinicalTrials.gov NCT00107497.
Conflict of interest statement
Ten-Year Results of FAST: A Randomized Controlled Trial of 5-Fraction Whole-Breast Radiotherapy for Early Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Adrian Murray Brunt
Peter Barrett-Lee
Andy Goodman
Adrian Harnett
Martin Hogg
Isabel Syndikus
Duncan Wheatley
Judith M. Bliss
No other potential conflicts of interest were reported.
Figures


Comment in
-
Hypofractionated Breast Irradiation: What's Next?J Clin Oncol. 2020 Oct 1;38(28):3245-3247. doi: 10.1200/JCO.20.01243. Epub 2020 Jul 14. J Clin Oncol. 2020. PMID: 32663118 No abstract available.
References
-
- Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: Long-term results of a randomised trial Radiother Oncol 759–172005 - PubMed
-
- Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: Long-term results of a randomised trial Lancet Oncol 7467–4712006 - PubMed
-
- Whelan TJ, Pignol J-P, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer N Engl J Med 362513–5202010 - PubMed
-
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials Lancet Oncol 141086–10942013 - PubMed
-
- National Institute for Health and Clinical Excellence: NICE clinical guideline 101: Early and locally advanced breast cancer: Diagnosis and management. http://www.nice.org.uk/guidance/ng101. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

