Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma
- PMID: 32663198
- PMCID: PMC7524481
- DOI: 10.1172/JCI137110
Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma
Abstract
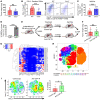
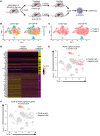
Immune checkpoint blockade (ICB) has revolutionized cancer therapeutics. Desmoplastic malignancies, such as cholangiocarcinoma (CCA), have an abundant tumor immune microenvironment (TIME). However, to date, ICB monotherapy in such malignancies has been ineffective. Herein, we identify tumor-associated macrophages (TAMs) as the primary source of programmed death-ligand 1 (PD-L1) in human and murine CCA. In a murine model of CCA, recruited PD-L1+ TAMs facilitated CCA progression. However, TAM blockade failed to decrease tumor progression due to a compensatory emergence of granulocytic myeloid-derived suppressor cells (G-MDSCs) that mediated immune escape by impairing T cell response. Single-cell RNA sequencing (scRNA-Seq) of murine tumor G-MDSCs highlighted a unique ApoE G-MDSC subset enriched with TAM blockade; further analysis of a human scRNA-Seq data set demonstrated the presence of a similar G-MDSC subset in human CCA. Finally, dual inhibition of TAMs and G-MDSCs potentiated ICB. In summary, our findings highlight the therapeutic potential of coupling ICB with immunotherapies targeting immunosuppressive myeloid cells in CCA.
Keywords: Cancer immunotherapy; Gastroenterology; Liver cancer; Macrophages; Oncology.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

