Cutting in-line with iron: ribosomal function and non-oxidative RNA cleavage
- PMID: 32663277
- PMCID: PMC7470983
- DOI: 10.1093/nar/gkaa586
Cutting in-line with iron: ribosomal function and non-oxidative RNA cleavage
Abstract
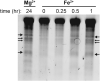
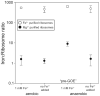
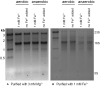
Divalent metal cations are essential to the structure and function of the ribosome. Previous characterizations of the ribosome performed under standard laboratory conditions have implicated Mg2+ as a primary mediator of ribosomal structure and function. Possible contributions of Fe2+ as a ribosomal cofactor have been largely overlooked, despite the ribosome's early evolution in a high Fe2+ environment, and the continued use of Fe2+ by obligate anaerobes inhabiting high Fe2+ niches. Here, we show that (i) Fe2+ cleaves RNA by in-line cleavage, a non-oxidative mechanism that has not previously been shown experimentally for this metal, (ii) the first-order in-line rate constant with respect to divalent cations is >200 times greater with Fe2+ than with Mg2+, (iii) functional ribosomes are associated with Fe2+ after purification from cells grown under low O2 and high Fe2+ and (iv) a small fraction of Fe2+ that is associated with the ribosome is not exchangeable with surrounding divalent cations, presumably because those ions are tightly coordinated by rRNA and deeply buried in the ribosome. In total, these results expand the ancient role of iron in biochemistry and highlight a possible new mechanism of iron toxicity.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Melnikov S., Ben-Shem A., Garreau de Loubresse N., Jenner L., Yusupova G., Yusupov M.. One core, two shells: bacterial and eukaryotic ribosomes. Nat. Struct. Mol. Biol. 2012; 19:560–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

