Reduced intensity conditioning for acute myeloid leukemia using melphalan- vs busulfan-based regimens: a CIBMTR report
- PMID: 32663298
- PMCID: PMC7362362
- DOI: 10.1182/bloodadvances.2019001266
Reduced intensity conditioning for acute myeloid leukemia using melphalan- vs busulfan-based regimens: a CIBMTR report
Erratum in
-
Zhou Z, Nath R, Cerny J, et al. Reduced intensity conditioning for acute myeloid leukemia using melphalan- vs busulfan-based regimens: a CIBMTR report. Blood Adv. 2020;4(13):3180-3190.Blood Adv. 2021 Jul 13;5(13):2752. doi: 10.1182/bloodadvances.2021005193. Blood Adv. 2021. PMID: 34242389 Free PMC article. No abstract available.
Abstract
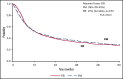
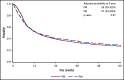
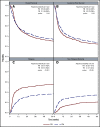
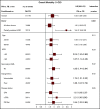
There is a lack of large comparative study on the outcomes of reduced intensity conditioning (RIC) in acute myeloid leukemia (AML) transplantation using fludarabine/busulfan (FB) and fludarabine/melphalan (FM) regimens. Adult AML patients from Center for International Blood and Marrow Transplant Research who received first RIC allo-transplant between 2001 and 2015 were studied. Patients were excluded if they received cord blood or identical twin transplant, total body irradiation in conditioning, or graft-versus-host disease (GVHD) prophylaxis with in vitro T-cell depletion. Primary outcome was overall survival (OS), secondary end points were leukemia-free survival (LFS), nonrelapse mortality (NRM), relapse, and GVHD. Multivariate survival model was used with adjustment for patient, leukemia, and transplant-related factors. A total of 622 patients received FM and 791 received FB RIC. Compared with FB, the FM group had fewer transplant in complete remission (CR), fewer matched sibling donors, and less usage of anti-thymocyte globulin or alemtuzumab. More patients in the FM group received marrow grafts and had transplantation before 2005. OS was significantly lower within the first 3 months posttransplant in the FM group (hazard ratio [HR] = 1.82, P < .001), but was marginally superior beyond 3 months (HR = 0.87, P = .05). LFS was better with FM compared with FB (HR = 0.89, P = .05). NRM was significantly increased in the FM group during the first 3 months of posttransplant (HR = 3.85, P < .001). Long-term relapse was lower with FM (HR = 0.65, P < .001). Analysis restricted to patients with CR showed comparable results. In conclusion, compared with FB, the FM RIC showed a marginally superior long-term OS and LFS and a lower relapse rate. A lower OS early posttransplant within 3 months was largely the result of a higher early NRM.
Conflict of interest statement
Conflict-of-interest disclosure: R.N. serves on the advisory boards of, was paid travel fees to the advisory board, and is a consultant for Actinum, Astellas, Daiichi Sankyo. N.N.S. has received honoraria and/or travel support from Incyte, Celgene, and Miltenyi Biotec; serves on scientific advisory boards for Kite, Celgene, and Cellectar; has equity ownership in Geron, Exelexis, Oncosec, and Cell Vault;, and has received institutional research support for clinical trials from BMS and Miltenyi Biotec. D.R. is an advisory board member for AbbVie, Agios, Jazz Novartis, Sanofi, Teva; a consultant advisory board member for AROG, Bayer, Pfizer; a speaker, advisory board member for Celgene and Gilead; and a speaker, advisory board member, consultant, and has received research funding from Stemline. I.P. is a data and safety monitoring board member for ExcellThera; and has received an educational grant from Merck. M.-A.P. is an ad hoc advisory board member for AbbVie, Bellicum, Bristol-Myers Squibb; a member of the scientific advisory board for MolMed and Nex-Immune; is a consultant for Merck; and has received research funding from Incyte (clinical trial) and Kite/Gilead (clinical trial). R.F.O. reports personal fees from AstraZeneca, outside the submitted work. M. Hertzberg receives speaker fees from and is on the advisory boards for Takeda and Roche; is on the advisory board for MSD; and receives speaker fees from Celgene. M.R.G. is a consultant for Agios, AbbVie, Amgen, Cardinal Health, Celgene, Incyte, Merck, Pfizer, Travagene, and Daischi Dankyo; owns stock in Medtronic; and receives research funding from Farma Therapeutics, Amgen, Genetech, Incyte, Janssen, and Novartis. U.G. receives speaker bureau and travel expense reimbursements from Incyte; research support, speaker bureau, and travel expense reimbursements from Jazz; speaker bureau and travel expense reimbursements from Astellas; and speaker bureau and travel expense reimbursements from Merck. A.T.G. receives grant/research support from Incyte, CTI Biopharma, and Sierra Oncology; and is on the advisory boards for PharmEssentia, CTI Biopharma, Promedior, Kartos, and Pfizer. M.D.L. received research grants from Pfizer and Celgene and is on the advisory boards for Kadmon, Pfizer, Incyte, and BMS. J.C. serves on the advisory boards for and receives travel expense reimbursements for Jazz Pharmaceuticals, Dallcal-Sankyo, and Incyte Inc. V.R.B. receives consulting fees from Rigel, Agios, Incyte, Omeros, and Takeda; has a partnership for health analytic research, LLC, and AbbVie; receives research funding (institutional) from Incyte, Jazz, Tolero Pharmaceuticals, Inc, and the National Marrow Donor Program; and receives drug support for a trial from Oncoceutics. A.A. is on the advisory board of Boston Biomedical and Incyte; receives research funding from Incyte; and is a consultant for Alpha Insights. B.M.S. is a consultant for Kiadis, Actinium Pharmaceutical, and Bristol-Meyers Squibb; has received research funding for clinical trials from Bellicum for clinical trials they sponsor; is a consultant for and has received funds from Actinium Pharmaceutical for trials they sponsor; and her spouse has equity in Oncoresponse, Inipharm, Blaze Bioscience, and AbbVie. G.L.U. is a consultant to Jaz, Astellas, and Genentech; and receives research funding from Macrogenics. The remaining authors declare no competing financial interests.
Figures

References
-
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813-1826. - PubMed
-
- McSweeney PA, Niederwieser D, Shizuru JA, et al. . Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390-3400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI126612/AI/NIAID NIH HHS/United States
- R21 HL140314/HL/NHLBI NIH HHS/United States
- R01 CA231141/CA/NCI NIH HHS/United States
- U01 HL128568/HL/NHLBI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 HL129472/HL/NHLBI NIH HHS/United States
- P01 CA111412/CA/NCI NIH HHS/United States
- R01 HL131731/HL/NHLBI NIH HHS/United States
- R01 HL126589/HL/NHLBI NIH HHS/United States
- R01 CA152108/CA/NCI NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U24 HL138660/HL/NHLBI NIH HHS/United States

