Current Status of COVID-19 (Pre)Clinical Vaccine Development
- PMID: 32663348
- PMCID: PMC7405471
- DOI: 10.1002/anie.202008319
Current Status of COVID-19 (Pre)Clinical Vaccine Development
Abstract
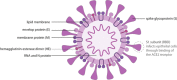
The current COVID-19 pandemic has a tremendous impact on daily life world-wide. Despite the ability to dampen the spread of SARS-CoV-2, the causative agent of the diseases, through restrictive interventions, it is believed that only effective vaccines will provide sufficient control over the disease and revert societal live back to normal. At present, a double-digit number of efforts are devoted to the development of a vaccine against COVID-19. Here, we provide an overview of these (pre)clinical efforts and provide background information on the technologies behind these vaccines. In addition, we discuss potential hurdles that need to be addressed prior to mass scale clinical translation of successful vaccine candidates.
Keywords: COVID-19; SARS-CoV-2; pandemic; vaccine.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous