Comparative efficacy of Chinese herbal injections combined with GP regimen chemotherapy for patients with advanced NSCLC: A protocol for systematic review and network meta-analysis
- PMID: 32664114
- PMCID: PMC7360220
- DOI: 10.1097/MD.0000000000021041
Comparative efficacy of Chinese herbal injections combined with GP regimen chemotherapy for patients with advanced NSCLC: A protocol for systematic review and network meta-analysis
Abstract
Background: Many research has indicated that some Chinese herb injections (CHIs) might be beneficial in combination with chemotherapy, however, with inconsistent results. Hence, the purpose of this network meta-analysis is to evaluate different CHIs plus cisplatin and gemcitabine (GP) with GP alone in terms of clinical efficacy and safety for treating patients with advanced NSCLC.
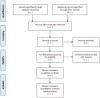
Methods: A comprehensive systematic search of clinical randomized controlled trials (RCTs) published in the PubMed, Embase, Web of Science (ISI), Cochrane Central Register of Controlled Trials (CENTRAL), China National Knowledge Infrastructure Database (CNKI), Chinese Scientific Journals Full-Text Database (VIP), Wanfang Database and China Biological Medicine Database (CBM) databases will be conducted to identify eligible studies up to the date of May 2020. The primary outcome measures objective response rate and adverse reactions (nausea and vomiting, leukopenia). The secondary outcome measures median survival time (MST), disease control rate, and quality of life. The methodological qualities, including the risk of bias, will be evaluated using the Cochrane risk of bias assessment tool, while confidence in the cumulative evidence will be evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. The network meta-analysis will be performed using WinBUGS 14 and Stata 15.1 software.
Results: Based on the current evidence, the potential rank of the efficacy and safety of CHIs plus GP chemotherapy for advanced NSCLC will be assessed, and a prioritization regimen will be summarized.
Conclusion: Evidence from this systematic review could be useful for patients, clinical practitioners, and guideline-makers to select an optimum proposal of CHIs plus GP for advanced NSCLC.
Ethics and dissemination: It is not necessary for ethical approval because it is based on published studies. The protocol will be disseminated in a peer-reviewed journal or presented at a topic-related conference.
Prospero registration number: CRD42020167142.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Comparative efficacy of Chinese herbal injections for treating severe pneumonia: A protocol for systematic review and Bayesian network meta-analysis of randomized controlled trials.PLoS One. 2022 May 23;17(5):e0262776. doi: 10.1371/journal.pone.0262776. eCollection 2022. PLoS One. 2022. PMID: 35604894 Free PMC article.
-
Network meta-analysis of Chinese herb injections combined with FOLFOX chemotherapy in the treatment of advanced colorectal cancer.J Clin Pharm Ther. 2016 Aug;41(4):383-91. doi: 10.1111/jcpt.12410. J Clin Pharm Ther. 2016. PMID: 27338003 Review.
-
Traditional Chinese medicine for mild-to-moderate ulcerative colitis: Protocol for a network meta-analysis of randomized controlled trials.Medicine (Baltimore). 2019 Aug;98(33):e16881. doi: 10.1097/MD.0000000000016881. Medicine (Baltimore). 2019. PMID: 31415431 Free PMC article.
-
Gemcitabine-based chemotherapy for advanced biliary tract carcinomas.Cochrane Database Syst Rev. 2018 Apr 6;4(4):CD011746. doi: 10.1002/14651858.CD011746.pub2. Cochrane Database Syst Rev. 2018. PMID: 29624208 Free PMC article.
Cited by
-
Chinese herbal medicine injections (CHMIs) for chronic pulmonary heart disease: A protocol for a Bayesian network meta-analysis.Medicine (Baltimore). 2021 Jan 22;100(3):e24128. doi: 10.1097/MD.0000000000024128. Medicine (Baltimore). 2021. PMID: 33546022 Free PMC article.
-
Seven oral traditional Chinese medicine combined with chemotherapy for the treatment of non-small cell lung cancer: a network meta-analysis.Pharm Biol. 2024 Dec;62(1):404-422. doi: 10.1080/13880209.2024.2351940. Epub 2024 May 13. Pharm Biol. 2024. PMID: 38739082 Free PMC article.
References
-
- Li Y, Guo QS. Research progress of maintenance therapy in advanced non-small cell lung cancer. Chin J Cancer Prev Treat 2014;21:800–4.
-
- Foss KM, Sima C, Ugolini D, et al. miR-1254 and miR-574-5p serum-based microRNA biomarkers for early-stage non-small cell lung cancer. J Thorac Oncol 2011;6:482–8. - PubMed
-
- Le CT. Adjuvant chemotherapy for resectable non-small cell lung cancer: where is it going? Ann Oncol 2010;21:196–8. - PubMed
-
- An ZJ, Guo CY, Chen MH, et al. Clinical observation of original chemotherapy for advanced non-small cell lung cancer between Changchun Swiss Bank combined with cisplatin regimen and docetaxel combined with cisplatin regimen. Chin J Med 2015;49:37–9.
-
- Huang TZ, Wang SZ, Yuan Y, et al. Analysis of related factors influencing survival time of patients with lung cancer. Chin Nurs Res 2016;30:4033–6.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical