A Review of T-Cell Related Therapy for Osteosarcoma
- PMID: 32664248
- PMCID: PMC7402310
- DOI: 10.3390/ijms21144877
A Review of T-Cell Related Therapy for Osteosarcoma
Abstract
Osteosarcoma is one of the most common primary malignant tumors of bone. The combination of chemotherapy and surgery makes the prognosis better than before, but therapy has not dramatically improved over the last three decades. This is partially because of the lack of a novel specialized drug for osteosarcoma, which is known as a tumor with heterogeneity. On the other hand, immunotherapy has been one of the most widely used strategies for many cancers over the last ten years. The therapies related to T-cell response, such as immune checkpoint inhibitor and chimeric antigen receptor T-cell therapy, are well-known options for some cancers. In this review, we offer the accumulated knowledge of T-cell-related immunotherapy for osteosarcoma, and discuss the future of the therapy.
Keywords: T-cell; immunotherapy; osteosarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

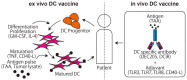
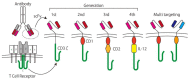
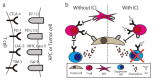

References
-
- Rosen G., Caparros B., Huvos A.G., Kosloff C., Nirenberg A., Cacavio A., Marcove R.C., Lane J.M., Mehta B., Urban C. Preoperative chemotherapy for osteogenic sarcoma: Selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::AID-CNCR2820490625>3.0.CO;2-E. - DOI - PubMed
-
- Italiano A., Mir O., Mathoulin-Pelissier S., Penel N., Piperno-Neumann S., Bompas E., Chevreau C., Duffaud F., Entz-Werlé N., Saada E., et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:446–455. doi: 10.1016/S1470-2045(19)30825-3. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

