Electrochemical Analysis and In Vitro Assay of Mg-0.5Ca-xY Biodegradable Alloys
- PMID: 32664267
- PMCID: PMC7411681
- DOI: 10.3390/ma13143082
Electrochemical Analysis and In Vitro Assay of Mg-0.5Ca-xY Biodegradable Alloys
Abstract
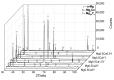
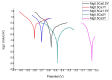
In recent years, biodegradable Mg-based materials have been increasingly studied to be used in the medical industry and beyond. A way to improve biodegradability rate in sync with the healing process of the natural human bone is to alloy Mg with other biocompatible elements. The aim of this research was to improve biodegradability rate and biocompatibility of Mg-0.5Ca alloy through addition of Y in 0.5/1.0/1.5/2.0/3.0wt.%. To characterize the chemical composition and microstructure of experimental Mg alloys, scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS), light microscopy (LM), and X-ray diffraction (XRD) were used. The linear polarization resistance (LPR) method was used to calculate corrosion rate as a measure of biodegradability rate. The cytocompatibility was evaluated by MTT assay (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide) and fluorescence microscopy. Depending on chemical composition, the dendritic α-Mg solid solution, as well as lamellar Mg2Ca and Mg24Y5 intermetallic compounds were found. The lower biodegradability rates were found for Mg-0.5Ca-2.0Y and Mg-0.5Ca-3.0Y which have correlated with values of cell viability. The addition of 2-3 wt.%Y in the Mg-0.5Ca alloy improved both the biodegradability rate and cytocompatibility behavior.
Keywords: Mg-Ca-Y alloys; electrochemical evaluation; in vitro test; microstructure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Rahim M.I., Ullah S., Mueller P. Advances and Challenges of Biodegradable Implant Materials with a Focus on Magnesium-Alloys and Bacterial Infections. Metals. 2018;8:532. doi: 10.3390/met8070532. - DOI
LinkOut - more resources
Full Text Sources

