High Mobility Group Box 1 in Human Cancer
- PMID: 32664328
- PMCID: PMC7407638
- DOI: 10.3390/cells9071664
High Mobility Group Box 1 in Human Cancer
Abstract
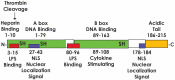
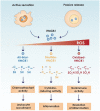
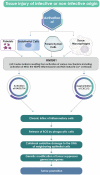
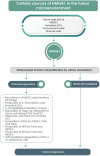
High mobility group box 1 (HMGB1) is an extremely versatile protein that is located predominantly in the nucleus of quiescent eukaryotic cells, where it is critically involved in maintaining genomic structure and function. During cellular stress, however, this multifaceted, cytokine-like protein undergoes posttranslational modifications that promote its translocation to the cytosol, from where it is released extracellularly, either actively or passively, according to cell type and stressor. In the extracellular milieu, HMGB1 triggers innate inflammatory responses that may be beneficial or harmful, depending on the magnitude and duration of release of this pro-inflammatory protein at sites of tissue injury. Heightened awareness of the potentially harmful activities of HMGB1, together with a considerable body of innovative, recent research, have revealed that excessive production of HMGB1, resulting from misdirected, chronic inflammatory responses, appears to contribute to all the stages of tumorigenesis. In the setting of established cancers, the production of HMGB1 by tumor cells per se may also exacerbate inflammation-related immunosuppression. These pro-inflammatory mechanisms of HMGB1-orchestrated tumorigenesis, as well as the prognostic potential of detection of elevated expression of this protein in the tumor microenvironment, represent the major thrusts of this review.
Keywords: T regulatory cells; Toll-like receptors; cytokines; immunosuppression; myeloid-derived suppressor cells; prognostic factor; receptor for advanced glycation end-products; redox isoforms; tumor microenvironment; tumorigenesis.
Conflict of interest statement
None of the authors has a conflict of interest to declare.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

