A Molecular Stratification of Chilean Gastric Cancer Patients with Potential Clinical Applicability
- PMID: 32664343
- PMCID: PMC7408697
- DOI: 10.3390/cancers12071863
A Molecular Stratification of Chilean Gastric Cancer Patients with Potential Clinical Applicability
Abstract
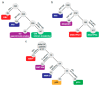
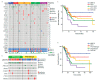
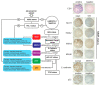
Gastric cancer (GC) is a complex and heterogeneous disease. In recent decades, The Cancer Genome Atlas (TCGA) and the Asian Cancer Research Group (ACRG) defined GC molecular subtypes. Unfortunately, these systems require high-cost and complex techniques and consequently their impact in the clinic has remained limited. Additionally, most of these studies are based on European, Asian, or North American GC cohorts. Herein, we report a molecular classification of Chilean GC patients into five subtypes, based on immunohistochemical (IHC) and in situ hybridization (ISH) methods. These were Epstein-Barr virus positive (EBV+), mismatch repair-deficient (MMR-D), epithelial to mesenchymal transition (EMT)-like, and accumulated (p53+) or undetected p53 (p53-). Given its lower costs this system has the potential for clinical applicability. Our results confirm relevant molecular alterations previously reported by TCGA and ACRG. We confirm EBV+ and MMR-D patients had the best prognosis and could be candidates for immunotherapy. Conversely, EMT-like displayed the poorest prognosis; our data suggest FGFR2 or KRAS could serve as potential actionable targets for these patients. Finally, we propose a low-cost step-by-step stratification system for GC patients. To the best of our knowledge, this is the first Latin American report on a molecular classification for GC. Pending further validation, this stratification system could be implemented into the routine clinic.
Keywords: Epstein–Barr virus; gastric cancer; molecular classification; molecular subtypes; targeted therapy.
Conflict of interest statement
B.N. is an advisor for Roche; J.M. is a consultant for Boehringer-Ingelheim and provides expert testimony to Roche; C.S. receives research funding from Roche and Novartis, and provides expert testimony for Pfizer and have received travel or accommodations, or expenses from Novartis and Pfizer; S.M. is a consultant for Foundation Medicine; E.K. participates at the speakers’ bureau for Novartis and have received travel support from Pfizer, Novartis and Roche Pharma; M.G. is an advisor for Merck Sharp & Dohme and Novartis, participates at the speakers’ bureau for Bristol-Myers & Squibb and Bayer. Also receives research funding from Bristol-Myers & Squibb (Inst) and Novartis (Inst). Also, has received travel accommodations and expenses from Roche. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Etemadi A., Safiri S., Sepanlou S.G., Ikuta K., Bisignano C., Shakeri R., Amani M., Fitzmaurice C., Nixon M., Abbasi N., et al. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. 2019;5:42–54. doi: 10.1016/S2468-1253(19)30328-0. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

