Ferroptosis in Liver Diseases: An Overview
- PMID: 32664576
- PMCID: PMC7404091
- DOI: 10.3390/ijms21144908
Ferroptosis in Liver Diseases: An Overview
Abstract
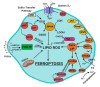
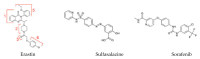
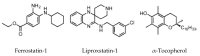
Ferroptosis is an iron-dependent form of cell death characterized by intracellular lipid peroxide accumulation and redox imbalance. Ferroptosis shows specific biological and morphological features when compared to the other cell death patterns. The loss of lipid peroxide repair activity by glutathione peroxidase 4 (GPX4), the presence of redox-active iron and the oxidation of polyunsaturated fatty acid (PUFA)-containing phospholipids are considered as distinct fingerprints of ferroptosis. Several pathways, including amino acid and iron metabolism, ferritinophagy, cell adhesion, p53, Keap1/Nrf2 and phospholipid biosynthesis, can modify susceptibility to ferroptosis. Through the decades, various diseases, including acute kidney injury; cancer; ischemia-reperfusion injury; and cardiovascular, neurodegenerative and hepatic disorders, have been associated with ferroptosis. In this review, we provide a comprehensive analysis of the main biological and biochemical mechanisms of ferroptosis and an overview of chemicals used as inducers and inhibitors. Then, we report the contribution of ferroptosis to the spectrum of liver diseases, acute or chronic. Finally, we discuss the use of ferroptosis as a therapeutic approach against hepatocellular carcinoma, the most common form of primary liver cancer.
Keywords: cell death; ferroptosis; iron metabolism; liver.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

