Deposition of NiO Nanoparticles on Nanosized Zeolite NaY for Production of Biofuel via Hydrogen-Free Deoxygenation
- PMID: 32664579
- PMCID: PMC7412304
- DOI: 10.3390/ma13143104
Deposition of NiO Nanoparticles on Nanosized Zeolite NaY for Production of Biofuel via Hydrogen-Free Deoxygenation
Abstract
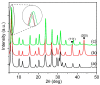
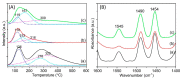
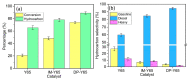
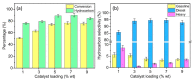
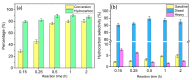
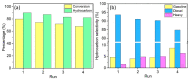
Nickel-based catalysts play an important role in the hydrogen-free deoxygenation for the production of biofuel. The yield and quality of the biofuel are critically affected by the physicochemical properties of NiO supported on nanosized zeolite Y (Y65, crystal size of 65 nm). Therefore, 10 wt% NiO supported on Y65 synthesized by using impregnation (IM) and deposition-precipitation (DP) methods were investigated. It was found that preparation methods have a significant effect on the deoxygenation of triolein. The initial rate of the DP method (14.8 goil·h-1) was 1.5 times higher than that of the IM method (9.6 goil·h-1). The DP-Y65 showed the best deoxygenation performance with a 80.0% conversion and a diesel selectivity of 93.7% at 380 °C within 1 h. The outstanding performance from the DP method was due to the smaller NiO particle size (3.57 ± 0.40 nm), high accessibility (H.F value of 0.084), and a higher Brönsted to Lewis acidity (B/L) ratio (0.29), which has improved the accessibility and deoxygenation ability of the catalyst. The NH4+ released from the decomposition of the urea during the DP process increased the B/L ratio of zeolite NaY. As a result, the pretreatment to convert Na-zeolite to H-zeolite in a conventional zeolite synthesis can be avoided. In this regard, the DP method offers a one-pot synthesis to produce smaller NiO-supported nanosized zeolite NaY with a high B/L ratio, and it managed to produce a higher yield with selectivity towards green diesel via deoxygenation under a hydrogen-free condition.
Keywords: NiO; deposition–precipitation; green diesel; triolein; zeolite Y.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- International Energy Agency (IEA) Global Energy & CO2 Status Report. International Energy Agency (IEA); Paris, France: 2018.
-
- U.S. Energy Information Administration Use of Energy Explained: Energy Use for Transportation 2019. [(accessed on 6 November 2019)]; Available online: https://www.eia.gov/energyexplained/use-of-energy/transportation.php.
-
- Hussein A.K. Applications of nanotechnology in renewable energies—A comprehensive overview and understanding. Renew. Sustain. Energy Rev. 2015;42:460–476. doi: 10.1016/j.rser.2014.10.027. - DOI
-
- Hanaki K., Portugal-Pereira J. The Effect of Biofuel Production on Greenhouse Gas Emission Reductions. In: Takeuchi K., Shiroyama H., Saito O., Matsuura M., editors. Biofuels and Sustainability: Holistic Perspectives for Policy-Making. Springer; Tokyo, Japan: 2018. pp. 53–71.
-
- Choo M.-Y., Oi L.E., Show P.L., Chang J.-S., Ling T.C., Ng E.-P., Phang S.M., Juan J.C. Recent progress in catalytic conversion of microalgae oil to green hydrocarbon: A review. J. Taiwan Inst. Chem. Eng. 2017;79(Suppl. C):116–124. doi: 10.1016/j.jtice.2017.06.028. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

