Fabrication and Plasma Surface Activation of Aligned Electrospun PLGA Fiber Fleeces with Improved Adhesion and Infiltration of Amniotic Epithelial Stem Cells Maintaining their Teno-inductive Potential
- PMID: 32664582
- PMCID: PMC7396982
- DOI: 10.3390/molecules25143176
Fabrication and Plasma Surface Activation of Aligned Electrospun PLGA Fiber Fleeces with Improved Adhesion and Infiltration of Amniotic Epithelial Stem Cells Maintaining their Teno-inductive Potential
Abstract
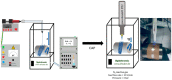
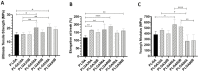
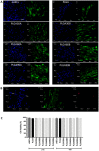
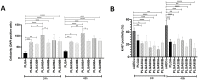
Electrospun PLGA microfibers with adequate intrinsic physical features (fiber alignment and diameter) have been shown to boost teno-differentiation and may represent a promising solution for tendon tissue engineering. However, the hydrophobic properties of PLGA may be adjusted through specific treatments to improve cell biodisponibility. In this study, electrospun PLGA with highly aligned microfibers were cold atmospheric plasma (CAP)-treated by varying the treatment exposure time (30, 60, and 90 s) and the working distance (1.3 and 1.7 cm) and characterized by their physicochemical, mechanical and bioactive properties on ovine amniotic epithelial cells (oAECs). CAP improved the hydrophilic properties of the treated materials due to the incorporation of new oxygen polar functionalities on the microfibers' surface especially when increasing treatment exposure time and lowering working distance. The mechanical properties, though, were affected by the treatment exposure time where the optimum performance was obtained after 60 s. Furthermore, CAP treatment did not alter oAECs' biocompatibility and improved cell adhesion and infiltration onto the microfibers especially those treated from a distance of 1.3 cm. Moreover, teno-inductive potential of highly aligned PLGA electrospun microfibers was maintained. Indeed, cells cultured onto the untreated and CAP treated microfibers differentiated towards the tenogenic lineage expressing tenomodulin, a mature tendon marker, in their cytoplasm. In conclusion, CAP treatment on PLGA microfibers conducted at 1.3 cm working distance represent the optimum conditions to activate PLGA surface by improving their hydrophilicity and cell bio-responsiveness. Since for tendon tissue engineering purposes, both high cell adhesion and mechanical parameters are crucial, PLGA treated for 60 s at 1.3 cm was identified as the optimal construct.
Keywords: PLGA; aligned microfibers; amniotic epithelial stem cells; cold atmospheric plasma; electrospinning; exposure time; tendon tissue engineering; working distance.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

