Irinotecan-Still an Important Player in Cancer Chemotherapy: A Comprehensive Overview
- PMID: 32664667
- PMCID: PMC7404108
- DOI: 10.3390/ijms21144919
Irinotecan-Still an Important Player in Cancer Chemotherapy: A Comprehensive Overview
Abstract
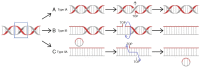
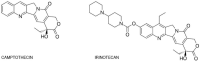
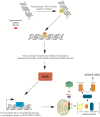
Irinotecan has been used in the treatment of various malignancies for many years. Still, the knowledge regarding this drug is expanding. The pharmacogenetics of the drug is the crucial component of response to irinotecan. Furthermore, new formulations of the drug are introduced in order to better deliver the drug and avoid potentially life-threatening side effects. Here, we give a comprehensive overview on irinotecan's molecular mode of action, metabolism, pharmacogenetics, and toxicity. Moreover, this article features clinically used combinations of the drug with other anticancer agents and introduces novel formulations of drugs (e.g., liposomal formulations, dendrimers, and nanoparticles). It also outlines crucial mechanisms of tumor cells' resistance to the active metabolite, ethyl-10-hydroxy-camptothecin (SN-38). We are sure that the article will constitute an important source of information for both new researchers in the field of irinotecan chemotherapy and professionals or clinicians who are interested in the topic.
Keywords: SN-38; drug combinations; drug resistance; irinotecan; new formulations; topoisomerases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

