Synthesis and Anticancer Activity of Dimeric Polyether Ionophores
- PMID: 32664671
- PMCID: PMC7408349
- DOI: 10.3390/biom10071039
Synthesis and Anticancer Activity of Dimeric Polyether Ionophores
Abstract
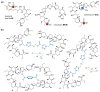
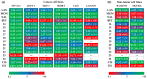
Polyether ionophores represent a group of natural lipid-soluble biomolecules with a broad spectrum of bioactivity, ranging from antibacterial to anticancer activity. Three seem to be particularly interesting in this context, namely lasalocid acid, monensin, and salinomycin, as they are able to selectively target cancer cells of various origin including cancer stem cells. Due to their potent biological activity and abundant availability, some research groups around the world have successfully followed semi-synthetic approaches to generate original derivatives of ionophores. However, a definitely less explored avenue is the synthesis and functional evaluation of their multivalent structures. Thus, in this paper, we describe the synthetic access to a series of original homo- and heterodimers of polyether ionophores, in which (i) two salinomycin molecules are joined through triazole linkers, or (ii) salinomycin is combined with lasalocid acid, monensin, or betulinic acid partners to form 'mixed' dimeric structures. Of note, all 11 products were tested in vitro for their antiproliferative activity against a panel of six cancer cell lines including the doxorubicin resistant colon adenocarcinoma LoVo/DX cell line; five dimers (14-15, 17-18 and 22) were identified to be more potent than the reference agents (i.e., both parent compound(s) and commonly used cytostatic drugs) in selective targeting of various types of cancer. Dimers 16 and 21 were also found to effectively overcome the resistance of the LoVo/DX cancer cell line.
Keywords: Huisgen 1,3-dipolar cycloaddition; SAR analysis; antiproliferative activity; betulinic acid; in vitro assay; multi-drug resistance; polyether ionophores; stereoselective reactions; tumor-specificity; ‘click’ chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

