Polymorphisms in Plasmodium vivax antifolate resistance markers in Afghanistan between 2007 and 2017
- PMID: 32664924
- PMCID: PMC7362531
- DOI: 10.1186/s12936-020-03319-0
Polymorphisms in Plasmodium vivax antifolate resistance markers in Afghanistan between 2007 and 2017
Abstract
Background: Plasmodium vivax is the predominant Plasmodium species in Afghanistan. National guidelines recommend the combination of chloroquine and primaquine (CQ-PQ) for radical treatment of P. vivax malaria. Artesunate in combination with the antifolates sulfadoxine-pyrimethamine (SP) has been first-line treatment for uncomplicated falciparum malaria until 2016. Although SP has been the recommended treatment for falciparum and not vivax malaria, exposure of the P. vivax parasite population to SP might still have been quite extensive because of community based management of malaria. The change in the P. vivax antifolate resistance markers between 2007 and 2017 were investigated.
Methods: Dried blood spots were collected (n = 185) from confirmed P. vivax patients in five malaria-endemic areas of Afghanistan bordering Tajikistan, Turkmenistan and Pakistan, including Takhar, Faryab, Laghman, Nangarhar, and Kunar, in 2007, 2010 and 2017. Semi-nested PCR, RFLP and nucleotide sequencing were used to assess the pyrimethamine resistant related mutations in P. vivax dihydrofolate reductase (pvdhfr I13L, P33L, N50I, F57L, S58R, T61I, S93H, S117N, I173L) and the sulfonamide resistance related mutations in P. vivax dihydropteroate synthase (pvdhps A383G, A553G).
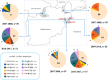
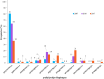
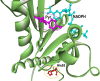
Results: In the 185 samples genotyped for pvdhfr and pvdhps mutations, 11 distinct haplotypes were observed, which evolved over time. In 2007, wild type pvdhfr and pvdhps were the most frequent haplotype in all study sites (81%, 80/99). However, in 2017, the frequency of the wild-type was reduced to 36%, (21/58; p value ≤ 0.001), with an increase in frequency of the double mutant pvdhfr and pvdhps haplotype S58RS117N (21%, 12/58), and the single pvdhfr mutant haplotype S117N (14%, 8/58). Triple and quadruple mutations were not found. In addition, pvdhfr mutations at position N50I (7%, 13/185) and the novel mutation S93H (6%, 11/185) were observed. Based on in silico protein modelling and molecular docking, the pvdhfr N50I mutation is expected to affect only moderately pyrimethamine binding, whereas the S93H mutation does not.
Conclusions: In the course of ten years, there has been a strong increase in the frequency pyrimethamine resistance related mutations in pvdhfr in the P. vivax population in Afghanistan, although triple and quadruple mutations conferring high grade resistance were not observed. This suggests relatively low drug pressure from SP on the P. vivax parasite population in the study areas. The impact of two newly identified mutations in the pvdhfr gene on pyrimethamine resistance needs further investigation.
Keywords: Afghanistan; Antifolate resistance; Dihydrofolate reductase (DHFR) and dihydropteroate synthase (DHPS); Plasmodium vivax.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- WHO . World malaria report 2018. Geneva: World Health Organization; 2018.
-
- Afghanistan Government . State of Afghan Cities 2015. Afghanistan Government: Kabul; 2015.
-
- Afghanistan Ministry of Health . National monitoring and evaluation plan 2018–2022. Afghanistan Ministry of Health: Kabul; 2018.
-
- Foote SJ, Cowman AF. The mode of action and the mechanism of resistance to antimalarial drugs. Acta Trop. 1994;56:157–171. - PubMed
-
- Peters W. Drug resistance in malaria parasites of animals and man. Adv Parasitol. 1998;41:1–62. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

