Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase II Trial
- PMID: 32665297
- PMCID: PMC7547532
- DOI: 10.1158/1078-0432.CCR-20-1695
Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase II Trial
Erratum in
-
Correction: Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-unrelated Head and Neck Cancer: A Multicenter, Phase II Trial.Clin Cancer Res. 2021 Jan 1;27(1):357. doi: 10.1158/1078-0432.CCR-20-4484. Clin Cancer Res. 2021. PMID: 33397681 No abstract available.
Abstract
Purpose: Pembrolizumab improved survival in patients with recurrent or metastatic head and neck squamous-cell carcinoma (HNSCC). The aims of this study were to determine if pembrolizumab would be safe, result in pathologic tumor response (pTR), and lower the relapse rate in patients with resectable human papillomavirus (HPV)-unrelated HNSCC.
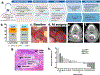
Patients and methods: Neoadjuvant pembrolizumab (200 mg) was administered and followed 2 to 3 weeks later by surgical tumor ablation. Postoperative (chemo)radiation was planned. Patients with high-risk pathology (positive margins and/or extranodal extension) received adjuvant pembrolizumab. pTR was quantified as the proportion of the resection bed with tumor necrosis, keratinous debris, and giant cells/histiocytes: pTR-0 (<10%), pTR-1 (10%-49%), and pTR-2 (≥50%). Coprimary endpoints were pTR-2 among all patients and 1-year relapse rate in patients with high-risk pathology (historical: 35%). Correlations of baseline PD-L1 and T-cell infiltration with pTR were assessed. Tumor clonal dynamics were evaluated (ClinicalTrials.gov NCT02296684).
Results: Thirty-six patients enrolled. After neoadjuvant pembrolizumab, serious (grades 3-4) adverse events and unexpected surgical delays/complications did not occur. pTR-2 occurred in eight patients (22%), and pTR-1 in eight other patients (22%). One-year relapse rate among 18 patients with high-risk pathology was 16.7% (95% confidence interval, 3.6%-41.4%). pTR ≥10% correlated with baseline tumor PD-L1, immune infiltrate, and IFNγ activity. Matched samples showed upregulation of inhibitory checkpoints in patients with pTR-0 and confirmed clonal loss in some patients.
Conclusions: Among patients with locally advanced, HPV-unrelated HNSCC, pembrolizumab was safe, and any pathologic response was observed in 44% of patients with 0% pathologic complete responses. The 1-year relapse rate in patients with high-risk pathology was lower than historical.
©2020 American Association for Cancer Research.
Figures


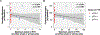

Comment in
-
Immunotherapy in Head and Neck Cancer-Ready for Prime Time or More Research Needed?Int J Radiat Oncol Biol Phys. 2021 Mar 1;109(3):647-650. doi: 10.1016/j.ijrobp.2020.11.022. Int J Radiat Oncol Biol Phys. 2021. PMID: 33516431 Free PMC article. No abstract available.
References
-
- Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. Massachusetts Medical Society; 2004;350:1937–44. - PubMed
-
- Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre J-L, Greiner RH, et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N Engl J Med. Massachusetts Medical Society; 2004;350:1945–52. - PubMed
-
- Harrington K, Temam S, Mehanna H, D’Cruz A, Jain M, D’Onofrio I, et al. Postoperative Adjuvant Lapatinib and Concurrent Chemoradiotherapy Followed by Maintenance Lapatinib Monotherapy in High-Risk Patients With Resected Squamous Cell Carcinoma of the Head and Neck: A Phase III, Randomized, Double-Blind, Placebo-Controlled Study. J Clin Oncol. 2015;33:4202–9. - PubMed
-
- Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn M-J, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–67. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

