Erythrocyte-driven immunization via biomimicry of their natural antigen-presenting function
- PMID: 32665441
- PMCID: PMC7395435
- DOI: 10.1073/pnas.2002880117
Erythrocyte-driven immunization via biomimicry of their natural antigen-presenting function
Abstract
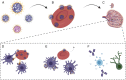
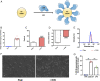
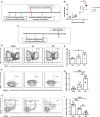
Erythrocytes naturally capture certain bacterial pathogens in circulation, kill them through oxidative stress, and present them to the antigen-presenting cells (APCs) in the spleen. By leveraging this innate immune function of erythrocytes, we developed erythrocyte-driven immune targeting (EDIT), which presents nanoparticles from the surface of erythrocytes to the APCs in the spleen. Antigenic nanoparticles were adsorbed on the erythrocyte surface. By engineering the number density of adsorbed nanoparticles, (i.e., the number of nanoparticles loaded per erythrocyte), they were predominantly delivered to the spleen rather than lungs, which is conventionally the target of erythrocyte-mediated delivery systems. Presentation of erythrocyte-delivered nanoparticles to the spleen led to improved antibody response against the antigen, higher central memory T cell response, and lower regulatory T cell response, compared with controls. Enhanced immune response slowed down tumor progression in a prophylaxis model. These findings suggest that EDIT is an effective strategy to enhance systemic immunity.
Keywords: biomimetic; erythrocyte hitchhiking; immunization; spleen targeting; vaccination.
Conflict of interest statement
Competing interest statement: A.U., Z.Z., and S.M. are inventors on a patent application that covers aspects of the technology described in this manuscript. The patent application is assigned to and managed by Harvard University.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

