Redefining fundamental concepts of transcription initiation in bacteria
- PMID: 32665585
- PMCID: PMC7990032
- DOI: 10.1038/s41576-020-0254-8
Redefining fundamental concepts of transcription initiation in bacteria
Abstract
Despite enormous progress in understanding the fundamentals of bacterial gene regulation, our knowledge remains limited when compared with the number of bacterial genomes and regulatory systems to be discovered. Derived from a small number of initial studies, classic definitions for concepts of gene regulation have evolved as the number of characterized promoters has increased. Together with discoveries made using new technologies, this knowledge has led to revised generalizations and principles. In this Expert Recommendation, we suggest precise, updated definitions that support a logical, consistent conceptual framework of bacterial gene regulation, focusing on transcription initiation. The resulting concepts can be formalized by ontologies for computational modelling, laying the foundation for improved bioinformatics tools, knowledge-based resources and scientific communication. Thus, this work will help researchers construct better predictive models, with different formalisms, that will be useful in engineering, synthetic biology, microbiology and genetics.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
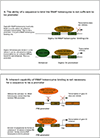

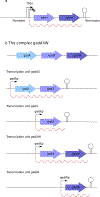
References
-
- Miller JH The operon. 7, (Cold Spring Harbor Laboratory Pr, 1980).
-
- Beckwith J The Operon: an Historical Account. in Escherichia coli and Salmonella: cellular and molecular biology (eds. Neidhardt F et al.) 1227–1231 (ASM Press, 1996).

