Plasma phospho-tau181 in presymptomatic and symptomatic familial Alzheimer's disease: a longitudinal cohort study
- PMID: 32665603
- PMCID: PMC7612227
- DOI: 10.1038/s41380-020-0838-x
Plasma phospho-tau181 in presymptomatic and symptomatic familial Alzheimer's disease: a longitudinal cohort study
Abstract
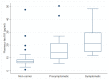
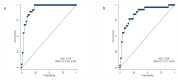
Blood biomarkers have great potential to advance clinical care and accelerate trials in Alzheimer's disease (AD). Plasma phospho-tau181 (p-tau181) is a promising blood biomarker however, it is unknown if levels increase in presymptomatic AD. Therefore, we investigated the timing of p-tau181 changes using 153 blood samples from 70 individuals in a longitudinal study of familial AD (FAD). Plasma p-tau181 was measured, using an in-house single molecule array assay. We compared p-tau181 between symptomatic carriers, presymptomatic carriers, and non-carriers, adjusting for age and sex. We examined the relationship between p-tau181 and neurofilament light and estimated years to/from symptom onset (EYO), as well as years to/from actual onset in a symptomatic subgroup. In addition, we studied associations between p-tau181 and clinical severity, as well testing for differences between genetic subgroups. Twenty-four were presymptomatic carriers (mean baseline EYO -9.6 years) while 27 were non-carriers. Compared with non-carriers, plasma p-tau181 concentration was higher in both symptomatic (p < 0.001) and presymptomatic mutation carriers (p < 0.001). Plasma p-tau181 showed considerable intra-individual variability but individual values discriminated symptomatic (AUC 0.93 [95% CI 0.85-0.98]) and presymptomatic (EYO ≥ -7 years) (AUC 0.86 [95% CI 0.72-0.94]) carriers from non-carriers of the same age and sex. From a fitted model there was evidence (p = 0.050) that p-tau181 concentrations were higher in mutation carriers than non-carriers from 16 years prior to estimated symptom onset. Our finding that plasma p-tau181 concentration is increased in symptomatic and presymptomatic FAD suggests potential utility as an easily accessible biomarker of AD pathology.
© 2020. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer’s disease: Report of the NINCDS-ADRDA work group★ under the auspices of department of health and human services task force on alzheimer’s disease. Neurology. 1984 Jul;34(7):939–44. - PubMed
-
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014 Jun 1;13(6):614–29. - PubMed
-
- Dubois B, Feldman HH, Jacova C, Cummings JL, DeKosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: A new lexicon. The Lancet Neurology. 2010;9:1118–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

