Region-specific mechanisms of corticosteroid-mediated inotropy in rat cardiomyocytes
- PMID: 32665640
- PMCID: PMC7360564
- DOI: 10.1038/s41598-020-68308-4
Region-specific mechanisms of corticosteroid-mediated inotropy in rat cardiomyocytes
Abstract
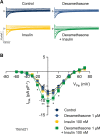
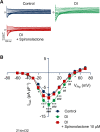
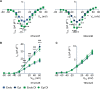
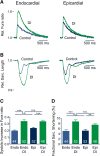
Regional differences in ion channel activity in the heart control the sequence of repolarization and may contribute to differences in contraction. Corticosteroids such as aldosterone or corticosterone increase the L-type Ca2+ current (ICaL) in the heart via the mineralocorticoid receptor (MR). Here, we investigate the differential impact of corticosteroid-mediated increase in ICaL on action potentials (AP), ion currents, intracellular Ca2+ handling and contractility in endo- and epicardial myocytes of the rat left ventricle. Dexamethasone led to a similar increase in ICaL in endocardial and epicardial myocytes, while the K+ currents Ito and IK were unaffected. However, AP duration (APD) and AP-induced Ca2+ influx (QCa) significantly increased exclusively in epicardial myocytes, thus abrogating the normal differences between the groups. Dexamethasone increased Ca2+ transients, contractility and SERCA activity in both regions, the latter possibly due to a decrease in total phospholamban (PLB) and an increase PLBpThr17. These results suggest that corticosteroids are powerful modulators of ICaL, Ca2+ transients and contractility in both endo- and epicardial myocytes, while APD and QCa are increased in epicardial myocytes only. This indicates that increased ICaL and SERCA activity rather than QCa are the primary drivers of contractility by adrenocorticoids.
Conflict of interest statement
The authors declare no competing interests.
Figures


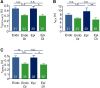
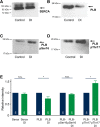
References
-
- Bandulik S. Of channels and pumps: different ways to boost the aldosterone? Acta Physiol. (Oxf.) 2017;220:332–360. - PubMed
-
- Pitt B, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999;341:709–717. - PubMed
-
- Pitt B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003;348:1309–1321. - PubMed
-
- Boixel C, Gavillet B, Rougier JS, Abriel H. Aldosterone increases voltage-gated sodium current in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2257–H2266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

