The roles of histone variants in fine-tuning chromatin organization and function
- PMID: 32665685
- PMCID: PMC8245300
- DOI: 10.1038/s41580-020-0262-8
The roles of histone variants in fine-tuning chromatin organization and function
Abstract
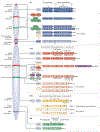
Histones serve to both package and organize DNA within the nucleus. In addition to histone post-translational modification and chromatin remodelling complexes, histone variants contribute to the complexity of epigenetic regulation of the genome. Histone variants are characterized by a distinct protein sequence and a selection of designated chaperone systems and chromatin remodelling complexes that regulate their localization in the genome. In addition, histone variants can be enriched with specific post-translational modifications, which in turn can provide a scaffold for recruitment of variant-specific interacting proteins to chromatin. Thus, through these properties, histone variants have the capacity to endow specific regions of chromatin with unique character and function in a regulated manner. In this Review, we provide an overview of recent advances in our understanding of the contribution of histone variants to chromatin function in mammalian systems. First, we discuss new molecular insights into chaperone-mediated histone variant deposition. Next, we discuss mechanisms by which histone variants influence chromatin properties such as nucleosome stability and the local chromatin environment both through histone variant sequence-specific effects and through their role in recruiting different chromatin-associated complexes. Finally, we focus on histone variant function in the context of both embryonic development and human disease, specifically developmental syndromes and cancer.
Figures



References
-
- Luger K, Mäder AW, Richmond RK, Sargent DF & Richmond TJ Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260 (1997). - PubMed
-
- Talbert PB & Henikoff S Histone variants — ancient wrap artists of the epigenome. Nat. Rev. Mol. Cell Biol 11, 264–275 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

