Polygenic risk scores and breast and epithelial ovarian cancer risks for carriers of BRCA1 and BRCA2 pathogenic variants
- PMID: 32665703
- PMCID: PMC7521995
- DOI: 10.1038/s41436-020-0862-x
Polygenic risk scores and breast and epithelial ovarian cancer risks for carriers of BRCA1 and BRCA2 pathogenic variants
Abstract
Purpose: We assessed the associations between population-based polygenic risk scores (PRS) for breast (BC) or epithelial ovarian cancer (EOC) with cancer risks for BRCA1 and BRCA2 pathogenic variant carriers.
Methods: Retrospective cohort data on 18,935 BRCA1 and 12,339 BRCA2 female pathogenic variant carriers of European ancestry were available. Three versions of a 313 single-nucleotide polymorphism (SNP) BC PRS were evaluated based on whether they predict overall, estrogen receptor (ER)-negative, or ER-positive BC, and two PRS for overall or high-grade serous EOC. Associations were validated in a prospective cohort.
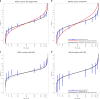
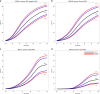
Results: The ER-negative PRS showed the strongest association with BC risk for BRCA1 carriers (hazard ratio [HR] per standard deviation = 1.29 [95% CI 1.25-1.33], P = 3×10-72). For BRCA2, the strongest association was with overall BC PRS (HR = 1.31 [95% CI 1.27-1.36], P = 7×10-50). HR estimates decreased significantly with age and there was evidence for differences in associations by predicted variant effects on protein expression. The HR estimates were smaller than general population estimates. The high-grade serous PRS yielded the strongest associations with EOC risk for BRCA1 (HR = 1.32 [95% CI 1.25-1.40], P = 3×10-22) and BRCA2 (HR = 1.44 [95% CI 1.30-1.60], P = 4×10-12) carriers. The associations in the prospective cohort were similar.
Conclusion: Population-based PRS are strongly associated with BC and EOC risks for BRCA1/2 carriers and predict substantial absolute risk differences for women at PRS distribution extremes.
Keywords: BRCA1/2; PRS; breast cancer; genetics; ovarian cancer.
Conflict of interest statement
G.P. has received honoraria from Novartis, Amgen, Roche, Pfizer, and AstraZeneca. The other authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA189867/CA/NCI NIH HHS/United States
- 11174/CRUK_/Cancer Research UK/United Kingdom
- UL1 TR002538/TR/NCATS NIH HHS/United States
- 20861/CRUK_/Cancer Research UK/United Kingdom
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 CA233191/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
- 23382/CRUK_/Cancer Research UK/United Kingdom
- 203477/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- P30 CA016672/CA/NCI NIH HHS/United States
- 26886/CRUK_/Cancer Research UK/United Kingdom
- P20 GM130423/GM/NIGMS NIH HHS/United States

