ATN status in amnestic and non-amnestic Alzheimer's disease and frontotemporal lobar degeneration
- PMID: 32666090
- PMCID: PMC7364757
- DOI: 10.1093/brain/awaa165
ATN status in amnestic and non-amnestic Alzheimer's disease and frontotemporal lobar degeneration
Abstract
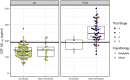
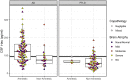
Under the ATN framework, CSF analytes provide evidence of the presence or absence of Alzheimer's disease pathological hallmarks: amyloid plaques (A), phosphorylated tau (T), and accompanying neurodegeneration (N). Still, differences in CSF levels across amnestic and non-amnestic variants or due to co-occurring pathologies might lead to misdiagnoses. We assess the diagnostic accuracy of CSF markers for amyloid, tau, and neurodegeneration in an autopsy cohort of 118 Alzheimer's disease patients (98 amnestic; 20 non-amnestic) and 64 frontotemporal lobar degeneration patients (five amnestic; 59 non-amnestic). We calculated between-group differences in CSF concentrations of amyloid-β1-42 peptide, tau protein phosphorylated at threonine 181, total tau, and the ratio of phosphorylated tau to amyloid-β1-42. Results show that non-amnestic Alzheimer's disease patients were less likely to be correctly classified under the ATN framework using independent, published biomarker cut-offs for positivity. Amyloid-β1-42 did not differ between amnestic and non-amnestic Alzheimer's disease, and receiver operating characteristic curve analyses indicated that amyloid-β1-42 was equally effective in discriminating both groups from frontotemporal lobar degeneration. However, CSF concentrations of phosphorylated tau, total tau, and the ratio of phosphorylated tau to amyloid-β1-42 were significantly lower in non-amnestic compared to amnestic Alzheimer's disease patients. Receiver operating characteristic curve analyses for these markers showed reduced area under the curve when discriminating non-amnestic Alzheimer's disease from frontotemporal lobar degeneration, compared to discrimination of amnestic Alzheimer's disease from frontotemporal lobar degeneration. In addition, the ATN framework was relatively insensitive to frontotemporal lobar degeneration, and these patients were likely to be classified as having normal biomarkers or biomarkers suggestive of primary Alzheimer's disease pathology. We conclude that amyloid-β1-42 maintains high sensitivity to A status, although with lower specificity, and this single biomarker provides better sensitivity to non-amnestic Alzheimer's disease than either the ATN framework or the phosphorylated-tau/amyloid-β1-42 ratio. In contrast, T and N status biomarkers differed between amnestic and non-amnestic Alzheimer's disease; standard cut-offs for phosphorylated tau and total tau may thus result in misclassifications for non-amnestic Alzheimer's disease patients. Consideration of clinical syndrome may help improve the accuracy of ATN designations for identifying true non-amnestic Alzheimer's disease.
Keywords: ATN; cerebrospinal fluid; frontotemporal degeneration; non-amnestic Alzheimer’s disease.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Andreasen N, Sjögren M, Blennow K.. CSF markers for Alzheimer’s disease: total tau, phospho-tau and Aβ42. World J Biol Psychiatry 2003; 4: 147–55. - PubMed
-
- Dubois B Feldman HH Jacova C Dekosky ST Barberger-Gateau P Cummings J, et al. . Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS–ADRDA criteria. The Lancet Neurology 2007; 6: 734–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

