Pharmacokinetics of micafungin in patients treated with extracorporeal membrane oxygenation: an observational prospective study
- PMID: 32667449
- PMCID: PMC7405733
- DOI: 10.5935/0103-507x.20200044
Pharmacokinetics of micafungin in patients treated with extracorporeal membrane oxygenation: an observational prospective study
Abstract
Objective: To determine micafungin plasma levels and pharmacokinetic behavior in patients treated with extracorporeal membrane oxygenation.
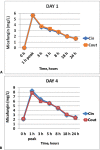
Methods: The samples were taken through an access point before and after the membrane in two tertiary hospitals in Spain. The times for the calculation of pharmacokinetic curves were before the administration of the drug and 1, 3, 5, 8, 18 and 24 hours after the beginning of the infusion on days one and four. The area under the curve, drug clearance, volume of distribution and plasma half-life time with a noncompartmental pharmacokinetic data analysis were calculated.
Results: The pharmacokinetics of the values analyzed on the first and fourth day of treatment did not show any concentration difference between the samples taken before the membrane (Cin) and those taken after the membrane (Cout), and the pharmacokinetic behavior was similar with different organ failures. The area under the curve (AUC) before the membrane on day 1 was 62.1 (95%CI 52.8 - 73.4) and the AUC after the membrane on this day was 63.4 (95%CI 52.4 - 76.7), p = 0.625. The AUC before the membrane on day 4 was 102.4 (95%CI 84.7 - 142.8) and the AUC was 100.9 (95%CI 78.2 - 138.8), p = 0.843.
Conclusion: The pharmacokinetic parameters of micafungin were not significantly altered.
Objetivo: Determinar os níveis plasmáticos e o comportamento farmacocinético da micafungina em pacientes tratados com oxigenação por membrana extracorpórea.
Métodos: As amostras foram colhidas por meio de pontos de acesso antes e depois da membrana, em dois hospitais espanhóis de nível terciário. Os momentos para o cálculo das curvas farmacocinéticas foram antes da administração do fármaco, e 1, 3, 5, 8, 18 e 24 horas após o início da infusão nos dias 1 e 4 de tratamento. Calcularam-se a área sob a curva, a depuração do fármaco, o volume de distribuição e a meia-vida plasmática por meio de análise farmacocinética não compartimental.
Resultados: Os valores farmacocinéticos analisados no primeiro e quarto dias de tratamento não mostram qualquer diferença de concentração entre amostras colhidas antes da membrana e após a membrana, e o comportamento farmacocinético foi similar na vigência de diferentes falências de órgãos. A área sob a curva antes da membrana no dia 1 foi de 62,1 (IC95% 52,8 - 73,4) e a área sob a curva após a membrana nesse mesmo dia foi de 63,4 (IC95% 52,4 - 76,7), com p = 0,625. A área sob a curva antes da membrana no dia 4 foi de 102,4 (IC95% 84,7 - 142,8), enquanto a área sob a curva após a membrana nesse mesmo dia foi de 100,9 (IC95% 78,2 - 138,8), com p = 0,843.
Conclusão: Os parâmetros farmacocinéticos da micafungina não foram alterados significantemente.
Conflict of interest statement
Figures
References
-
- Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306(15):1659–1668. - PubMed
-
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, et al. Extracorporeal membrane oxygenation for 2009 Influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895. - PubMed
-
- Rehder KJ, Turner DA, Bonadonna D, Walczak RJ, Rudder RJ, Cheifetz IM. Technological advances in extracorporeal membrane oxygenation for respiratory failure. Expert Rev Respir Med. 2012;6(4):377–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources