Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US
- PMID: 32667668
- PMCID: PMC7364338
- DOI: 10.1001/jamainternmed.2020.3596
Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US
Erratum in
-
Error in Key Points and Omission of Collaborators in Article Information.JAMA Intern Med. 2020 Nov 1;180(11):1555. doi: 10.1001/jamainternmed.2020.4568. JAMA Intern Med. 2020. PMID: 32780843 Free PMC article. No abstract available.
-
Errors in Reported SOFA Scores and Added Supplement Listing STOP-COVID Investigators.JAMA Intern Med. 2021 Aug 1;181(8):1144. doi: 10.1001/jamainternmed.2021.0317. JAMA Intern Med. 2021. PMID: 33900368 Free PMC article. No abstract available.
Abstract
Importance: The US is currently an epicenter of the coronavirus disease 2019 (COVID-19) pandemic, yet few national data are available on patient characteristics, treatment, and outcomes of critical illness from COVID-19.
Objectives: To assess factors associated with death and to examine interhospital variation in treatment and outcomes for patients with COVID-19.
Design, setting, and participants: This multicenter cohort study assessed 2215 adults with laboratory-confirmed COVID-19 who were admitted to intensive care units (ICUs) at 65 hospitals across the US from March 4 to April 4, 2020.
Exposures: Patient-level data, including demographics, comorbidities, and organ dysfunction, and hospital characteristics, including number of ICU beds.
Main outcomes and measures: The primary outcome was 28-day in-hospital mortality. Multilevel logistic regression was used to evaluate factors associated with death and to examine interhospital variation in treatment and outcomes.
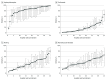
Results: A total of 2215 patients (mean [SD] age, 60.5 [14.5] years; 1436 [64.8%] male; 1738 [78.5%] with at least 1 chronic comorbidity) were included in the study. At 28 days after ICU admission, 784 patients (35.4%) had died, 824 (37.2%) were discharged, and 607 (27.4%) remained hospitalized. At the end of study follow-up (median, 16 days; interquartile range, 8-28 days), 875 patients (39.5%) had died, 1203 (54.3%) were discharged, and 137 (6.2%) remained hospitalized. Factors independently associated with death included older age (≥80 vs <40 years of age: odds ratio [OR], 11.15; 95% CI, 6.19-20.06), male sex (OR, 1.50; 95% CI, 1.19-1.90), higher body mass index (≥40 vs <25: OR, 1.51; 95% CI, 1.01-2.25), coronary artery disease (OR, 1.47; 95% CI, 1.07-2.02), active cancer (OR, 2.15; 95% CI, 1.35-3.43), and the presence of hypoxemia (Pao2:Fio2<100 vs ≥300 mm Hg: OR, 2.94; 95% CI, 2.11-4.08), liver dysfunction (liver Sequential Organ Failure Assessment score of 2-4 vs 0: OR, 2.61; 95% CI, 1.30-5.25), and kidney dysfunction (renal Sequential Organ Failure Assessment score of 4 vs 0: OR, 2.43; 95% CI, 1.46-4.05) at ICU admission. Patients admitted to hospitals with fewer ICU beds had a higher risk of death (<50 vs ≥100 ICU beds: OR, 3.28; 95% CI, 2.16-4.99). Hospitals varied considerably in the risk-adjusted proportion of patients who died (range, 6.6%-80.8%) and in the percentage of patients who received hydroxychloroquine, tocilizumab, and other treatments and supportive therapies.
Conclusions and relevance: This study identified demographic, clinical, and hospital-level risk factors that may be associated with death in critically ill patients with COVID-19 and can facilitate the identification of medications and supportive therapies to improve outcomes.
Conflict of interest statement
Figures

References
-
- Johns Hopkins University Coronavirus Resource Center. Accessed June 19, 2020. https://coronavirus.jhu.edu/
Publication types
MeSH terms
Grants and funding
- K08 GM134220/GM/NIGMS NIH HHS/United States
- F32 HL149337/HL/NHLBI NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
- R03 AG060179/AG/NIA NIH HHS/United States
- R01 HL153384/HL/NHLBI NIH HHS/United States
- L60 DK126281/DK/NIDDK NIH HHS/United States
- K23 DK124644/DK/NIDDK NIH HHS/United States
- K23 HL130648/HL/NHLBI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- K23 DK116967/DK/NIDDK NIH HHS/United States
- L30 HL149016/HL/NHLBI NIH HHS/United States
- K23 DK120811/DK/NIDDK NIH HHS/United States
- UL1 TR002389/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

