Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity
- PMID: 32667831
- PMCID: PMC7499617
- DOI: 10.1200/JCO.19.02103
Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity
Abstract
Purpose: Axicabtagene ciloleucel (axi-cel) was approved by the Food and Drug Administration for relapsed aggressive B-cell non-Hodgkin lymphoma in part on the basis of durable remission rates of approximately 40% in a clinical trial population. Whether this efficacy, and the rates of toxicity, would be consistent in a postcommercial setting, with relaxed eligibility criteria and bridging therapy, is unknown. This study describes the efficacy and safety correlates and outcomes in this setting.
Patients and methods: One hundred twenty-two patients from 7 medical centers in the United States were treated with axi-cel and were included in a modified intent-to-treat (mITT) analysis. Seventy-six patients (62%) were ineligible for the ZUMA-1 trial. Response and toxicity rates, duration of response (DOR), survival, and covariates are described on the basis of the mITT population. Correlative studies on blood and tumor samples were performed to investigate potential biomarkers of response and resistance.
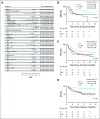
Results: Median follow-up was 10.4 months. In the mITT population, the best overall and complete response (CR) rates were 70% and 50%, respectively. Median DOR and progression-free survival (PFS) were 11.0 and 4.5 months in all patients and were not reached (NR) in CR patients. Median overall survival (OS) was NR; 1-year OS was 67% (95% CI, 59% to 77%). Although response rates were similar in the ZUMA-1-eligible and ZUMA-1-ineligible groups (70% v 68%), there was a statistically significant improvement in CR rate (63% v 42%, P = .016), DOR (median, NR v 5.0 months; P = .014), PFS (median, NR v 3.3 months; P = .020), and OS (1-year OS, 89% v 54%; P < .001) in patients who were ZUMA-1 eligible. Rates of grade ≥ 3 cytokine release syndrome and neurotoxicty were 16% and 35%, respectively.
Conclusion: Axi-cel yields similar rates of overall response and toxicity in commercial and trial settings, although CR rates and DOR were more favorable in patients eligible for ZUMA-1.
Figures





Comment in
-
Early Off-Study Experience of Chimeric Antigen Receptor T Cells in Aggressive Lymphoma: Closer to a Real-World Setting.J Clin Oncol. 2020 Sep 20;38(27):3085-3087. doi: 10.1200/JCO.20.01134. Epub 2020 Jul 15. J Clin Oncol. 2020. PMID: 32667832 No abstract available.
References
-
- Seshadri T, Stakiw J, Pintilie M, et al. Utility of subsequent conventional dose chemotherapy in relapsed/refractory transplant-eligible patients with diffuse large B-cell lymphoma failing platinum-based salvage chemotherapy. Hematology. 2008;13:261–266. - PubMed
-
- Hitz F, Connors JM, Gascoyne RD, et al. Outcome of patients with primary refractory diffuse large B cell lymphoma after R-CHOP treatment. Ann Hematol. 2015;94:1839–1843. - PubMed
-
- Nagle SJ, Woo K, Schuster SJ, et al. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol. 2013;88:890–894. - PubMed
-
- Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51:51–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

