A next generation vaccine against human rabies based on a single dose of a chimpanzee adenovirus vector serotype C
- PMID: 32667913
- PMCID: PMC7363076
- DOI: 10.1371/journal.pntd.0008459
A next generation vaccine against human rabies based on a single dose of a chimpanzee adenovirus vector serotype C
Erratum in
-
Correction: A next generation vaccine against human rabies based on a single dose of a chimpanzee adenovirus vector serotype C.PLoS Negl Trop Dis. 2021 Apr 13;15(4):e0009348. doi: 10.1371/journal.pntd.0009348. eCollection 2021 Apr. PLoS Negl Trop Dis. 2021. PMID: 33848297 Free PMC article.
Abstract
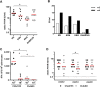
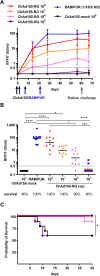
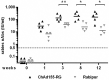
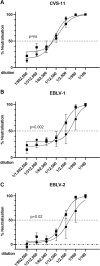
Rabies, caused by RNA viruses in the Genus Lyssavirus, is the most fatal of all infectious diseases. This neglected zoonosis remains a major public health problem in developing countries, causing the death of an estimated 25,000-159,000 people each year, with more than half of them in children. The high incidence of human rabies in spite of effective vaccines is mainly linked to the lack of compliance with the complicated administration schedule, inadequacies of the community public health system for local administration by the parenteral route and the overall costs of the vaccine. The goal of our work was the development of a simple, affordable and effective vaccine strategy to prevent human rabies virus infection. This next generation vaccine is based on a replication-defective chimpanzee adenovirus vector belonging to group C, ChAd155-RG, which encodes the rabies glycoprotein (G). We demonstrate here that a single dose of this vaccine induces protective efficacy in a murine model of rabies challenge and elicits strong and durable neutralizing antibody responses in vaccinated non-human primates. Importantly, we demonstrate that one dose of a commercial rabies vaccine effectively boosts the neutralizing antibody responses induced by ChAd155-RG in vaccinated monkeys, showing the compatibility of the novel vectored vaccine with the current post-exposure prophylaxis in the event of rabies virus exposure. Finally, we demonstrate that antibodies induced by ChAd155-RG can also neutralize European bat lyssaviruses 1 and 2 (EBLV-1 and EBLV-2) found in bat reservoirs.
Conflict of interest statement
Benjamin Wizel is an employee of the GSK group of companies.
Figures




References
-
- World Health Organization, Savioli L, Daumerie D, World Health Organization. Department of Control of Neglected Tropical Diseases. Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected tropical diseases. Geneva, Switzerland: World Health Organization; 2013. xii, 138 pages p.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

