Mechanical Heterogeneity in the Bone Microenvironment as Characterized by Atomic Force Microscopy
- PMID: 32668233
- PMCID: PMC7401034
- DOI: 10.1016/j.bpj.2020.06.026
Mechanical Heterogeneity in the Bone Microenvironment as Characterized by Atomic Force Microscopy
Abstract
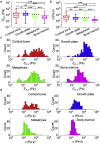
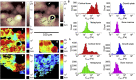
Bones are structurally heterogeneous organs with diverse functions that undergo mechanical stimuli across multiple length scales. Mechanical characterization of the bone microenvironment is important for understanding how bones function in health and disease. Here, we describe the mechanical architecture of cortical bone, the growth plate, metaphysis, and marrow in fresh murine bones, probed using atomic force microscopy in physiological buffer. Both elastic and viscoelastic properties are found to be highly heterogeneous with moduli ranging over three to five orders of magnitude, both within and across regions. All regions include extremely compliant areas, with moduli of a few pascal and viscosities as low as tens of Pa·s. Aging impacts the viscoelasticity of the bone marrow strongly but has a limited effect on the other regions studied. Our approach provides the opportunity to explore the mechanical properties of complex tissues at the length scale relevant to cellular processes and how these impact aging and disease.
Copyright © 2020. Published by Elsevier Inc.
Figures



References
-
- Bussard K.M., Gay C.V., Mastro A.M. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55. - PubMed
-
- Morgan E.F., Barnes G.L., Einhorn T.A. The bone organ system: form and function. In: Marcus R., Feldman D., Dempster D.W., Luckey M., Cauley J.A., editors. Osteoporosis. Academic Press; 2013. pp. 3–20.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

