Interferon-Induced Transmembrane Protein 1 (IFITM1) Promotes Distant Metastasis of Small Cell Lung Cancer
- PMID: 32668617
- PMCID: PMC7404048
- DOI: 10.3390/ijms21144934
Interferon-Induced Transmembrane Protein 1 (IFITM1) Promotes Distant Metastasis of Small Cell Lung Cancer
Abstract
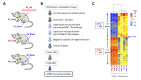
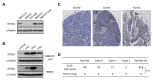
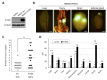
Small cell lung cancer (SCLC) is a severe malignancy associated with early and widespread metastasis. To study SCLC metastasis, we previously developed an orthotopic transplantation model using the human SCLC cell line DMS273. In the model, metastatic foci were found in distant tissues such as bone and the adrenal gland, similarly as observed in patients with SCLC. In this study, we evaluated the differentially expressed genes between orthotopic and metastatic tumors in the model. We isolated tumor cells from orthotopic and metastatic sites, and the tumor cell RNA was analyzed using DNA microarray analysis. We found that 19 genes in metastatic tumors were upregulated by more than 4-fold compared with their expression in orthotopic tumors. One of these genes encodes a transmembrane protein, interferon (IFN)-induced transmembrane protein 1 (IFITM1), and immunohistochemical analysis confirmed the higher expression of the protein in metastatic sites than in orthotopic sites. IFITM1 was also detected in some SCLC cell lines and lung tumors from patients with SCLC. The overexpression of IFITM1 in DMS273 cells increased their metastatic formation in the orthotopic model and in an experimental metastasis model. Conversely, the silencing of IFITM1 suppressed metastatic formation by DMS273 cells. We also found that IFITM1 overexpression promoted the metastatic formation of NCI-H69 human SCLC cells. These results demonstrate that IFITM1 promotes distant metastasis in xenograft models of human SCLC.
Keywords: IFITM1; experimental metastasis model; metastasis; orthotopic transplantation model; small cell lung cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kuramoto T., Goto H., Mitsuhashi A., Tabata S., Ogawa H., Uehara H., Saijo A., Kakiuchi S., Maekawa Y., Yasutomo K., et al. Dll4-Fc, an inhibitor of Dll4-notch signaling, suppresses liver metastasis of small cell lung cancer cells through the downregulation of the NF-kappaB activity. Mol. Cancer Ther. 2012;11:2578–2587. doi: 10.1158/1535-7163.MCT-12-0640. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

