Copper Homeostasis in Mammals, with Emphasis on Secretion and Excretion. A Review
- PMID: 32668621
- PMCID: PMC7403968
- DOI: 10.3390/ijms21144932
Copper Homeostasis in Mammals, with Emphasis on Secretion and Excretion. A Review
Abstract
One of the hallmarks of Cu metabolism in mammals is that tissue and fluid levels are normally maintained within a very narrow range of concentrations. This results from the ability of the organism to respond to variations in intake from food and drink by balancing excretion, which occurs mainly via the bile and feces. Although this sounds straightforward and we have already learned a great deal about aspects of this process, the balance between overall intake and excretion occurs over a high background of Cu recycling, which has generally been ignored. In fact, most of the Cu absorbed from the GI tract actually comes from digestive fluids and is constantly "re-used". A great deal more recycling of Cu probably occurs in the interior, between cells of individual tissues and the fluid of the blood and interstitium. This review presents what is known that is pertinent to understanding these complexities of mammalian Cu homeostasis and indicates where further studies are needed.
Keywords: ATP7A; ATP7B; bile; ceruloplasmin; copper; excretion; gastric fluid; kidney; liver; pancreatic fluid; saliva; secretion; small copper carriers; urine.
Conflict of interest statement
The author declares no conflict of interest.
Figures


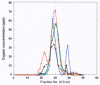


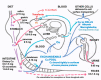
References
-
- Linder M.C. Biochemistry of Copper. Plenum; New York, NY, USA: 1991.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

