4,5-Diazafluorene and 9,9'-Dimethyl-4,5-Diazafluorene as Ligands Supporting Redox-Active Mn and Ru Complexes
- PMID: 32668660
- PMCID: PMC7396985
- DOI: 10.3390/molecules25143189
4,5-Diazafluorene and 9,9'-Dimethyl-4,5-Diazafluorene as Ligands Supporting Redox-Active Mn and Ru Complexes
Abstract
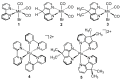
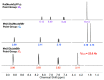
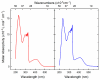
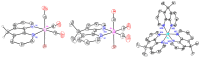
4,5-diazafluorene (daf) and 9,9'-dimethyl-4,5-diazafluorene (Me2daf) are structurally similar to the important ligand 2,2'-bipyridine (bpy), but significantly less is known about the redox and spectroscopic properties of metal complexes containing Me2daf as a ligand than those containing bpy. New complexes Mn(CO)3Br(daf) (2), Mn(CO)3Br(Me2daf) (3), and [Ru(Me2daf)3](PF6)2 (5) have been prepared and fully characterized to understand the influence of the Me2daf framework on their chemical and electrochemical properties. Structural data for 2, 3, and 5 from single-crystal X-ray diffraction analysis reveal a distinctive widening of the daf and Me2daf chelate angles in comparison to the analogous Mn(CO)3(bpy)Br (1) and [Ru(bpy)3]2+ (4) complexes. Electronic absorption data for these complexes confirm the electronic similarity of daf, Me2daf, and bpy, as spectra are dominated in each case by metal-to-ligand charge transfer bands in the visible region. However, the electrochemical properties of 2, 3, and 5 reveal that the redox-active Me2daf framework in 3 and 5 undergoes reduction at a slightly more negative potential than that of bpy in 1 and 4. Taken together, the results indicate that Me2daf could be useful for preparation of a variety of new redox-active compounds, as it retains the useful redox-active nature of bpy but lacks the acidic, benzylic C-H bonds that can induce secondary reactivity in complexes bearing daf.
Keywords: 4,5-diazafluorene; 9,9’-dimethyl-4,5-diazafluorene; electrochemistry; manganese tricarbonyl; ruthenium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


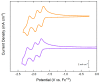
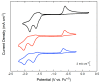
References
-
- English A.M., Delaive P.J., Gray H.B., Lum V.R. Metalloprotein electron-transfer mechanisms. Quenching of electronically excited tris(2,2’-bipyridine)ruthenium(II) by reduced blue copper proteins. J. Am. Chem. Soc. 1982;104:870–871. doi: 10.1021/ja00367a047. - DOI
-
- Brunschwig B.S., Delaive P.J., English A.M., Goldberg M., Gray H.B., Mayo S.L., Sutin N. Kinetics and mechanisms of electron transfer between blue copper proteins and electronically excited chromium and ruthenium polypyridine complexes. Inorg. Chem. 1985;24:3743–3749. doi: 10.1021/ic00217a010. - DOI
-
- Sullivan B.P., Bolinger C.M., Conrad D., Vining W.J., Meyer T.J. One- and two-electron pathways in the electrocatalytic reduction of CO2 by fac-Re(bpy)(CO)3Cl (bpy = 2,2’-bipyridine) J. Chem. Soc. Chem. Commun. 1985;1414 doi: 10.1039/c39850001414. - DOI
-
- Meyer T.J. Electron Transfer Reactions Induced by Excited State Quenching. Isr. J. Chem. 1976;15:200–205. doi: 10.1002/ijch.197600036. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

