Sphingosine-1-Phosphate Metabolism in the Regulation of Obesity/Type 2 Diabetes
- PMID: 32668665
- PMCID: PMC7407406
- DOI: 10.3390/cells9071682
Sphingosine-1-Phosphate Metabolism in the Regulation of Obesity/Type 2 Diabetes
Abstract
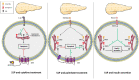
Obesity is a pathophysiological condition where excess free fatty acids (FFA) target and promote the dysfunctioning of insulin sensitive tissues and of pancreatic β cells. This leads to the dysregulation of glucose homeostasis, which culminates in the onset of type 2 diabetes (T2D). FFA, which accumulate in these tissues, are metabolized as lipid derivatives such as ceramide, and the ectopic accumulation of the latter has been shown to lead to lipotoxicity. Ceramide is an active lipid that inhibits the insulin signaling pathway as well as inducing pancreatic β cell death. In mammals, ceramide is a key lipid intermediate for sphingolipid metabolism as is sphingosine-1-phosphate (S1P). S1P levels have also been associated with the development of obesity and T2D. In this review, the current knowledge on S1P metabolism in regulating insulin signaling in pancreatic β cell fate and in the regulation of feeding by the hypothalamus in the context of obesity and T2D is summarized. It demonstrates that S1P can display opposite effects on insulin sensitive tissues and pancreatic β cells, which depends on its origin or its degradation pathway.
Keywords: Sphingosine-1-phosphate; hypothalamus; insulin resistance; obesity; pancreatic β cell fate; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

