An Infant Formula with Partially Hydrolyzed Whey Protein Supports Adequate Growth and Is Safe and Well-Tolerated in Healthy, Term Infants: A Randomized, Double-Blind, Equivalence Trial
- PMID: 32668666
- PMCID: PMC7400250
- DOI: 10.3390/nu12072072
An Infant Formula with Partially Hydrolyzed Whey Protein Supports Adequate Growth and Is Safe and Well-Tolerated in Healthy, Term Infants: A Randomized, Double-Blind, Equivalence Trial
Abstract
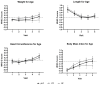
The current study evaluates the safety and tolerance of a partially hydrolyzed whey protein-based infant formula (PHF) versus an in intact cow's milk protein formula (IPF). Breastfed infants were included as a reference group. In a multi-country, multicenter, randomized, double-blinded, controlled clinical trial, infants whose mothers intended to fully formula feed were randomized to PHF (n = 134) or IPF (n = 134) from ≤14 days to 17 weeks of age. The equivalence analysis of weight gain per day within margins of +/-3 g/d (primary outcome), the recorded adverse events, growth and gastro-intestinal tolerance parameters were considered for the safety evaluation. Equivalence of weight gain per day from enrolment until 17 weeks of age was demonstrated in the PHF group compared to the IPF group (difference in means -1.2 g/d; 90% CI (-2.42; 0.02)), with estimated means (SE) of 30.2 (0.5) g/d and 31.4 (0.5) g/d, respectively. No significant differences in growth outcomes, the number, severity or type of (serious) adverse events and tolerance outcomes, were observed between the two formula groups. A partially hydrolyzed whey protein-based infant formula supports adequate infant growth, with a daily weight gain equivalent to a standard intact protein-based formula; it is also safe for use and well-tolerated in healthy term infants.
Keywords: infant growth; partially hydrolyzed formula; prebiotic; safety; tolerance.
Conflict of interest statement
M.A.B., A.R. and E.H. are employees of Danone Nutricia Research and have been involved in the design of the study, the analyses and interpretation of the data and the writing of the manuscript. None of the other authors have a conflict of interest. The study was sponsored by Danone Nutricia Research, which provided the infant formulas and financial support to the participating centers in order to conduct the study.
Figures

References
-
- EFSA. Panel on Dietetic Products. Nutrition and Allergies (NDA) Scientific opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014;12:3760. doi: 10.2903/j.efsa.2014.3760. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

