CCR2 of Tumor Microenvironmental Cells Is a Relevant Modulator of Glioma Biology
- PMID: 32668709
- PMCID: PMC7408933
- DOI: 10.3390/cancers12071882
CCR2 of Tumor Microenvironmental Cells Is a Relevant Modulator of Glioma Biology
Abstract
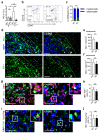
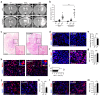
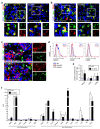
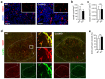
Glioblastoma multiforme (GBM) shows a high influx of tumor-associated macrophages (TAMs). The CCR2/CCL2 pathway is considered a relevant signal for the recruitment of TAMs and has been suggested as a therapeutic target in malignant gliomas. We found that TAMs of human GBM specimens and of a syngeneic glioma model express CCR2 to varying extents. Using a Ccr2-deficient strain for glioma inoculation revealed a 30% reduction of TAMs intratumorally. This diminished immune cell infiltration occurred with augmented tumor volumes likely based on increased cell proliferation. Remaining TAMs in Ccr2-/- mice showed comparable surface marker expression patterns in comparison to wildtype mice, but expression levels of inflammatory transcription factors (Stat3, Irf7, Cox2) and cytokines (Ifnβ, Il1β, Il12α) were considerably affected. Furthermore, we demonstrated an impact on blood vessel integrity, while vascularization of tumors appeared similar between mouse strains. The higher stability and attenuated leakiness of the tumor vasculature imply improved sustenance of glioma tissue in Ccr2-/- mice. Additionally, despite TAMs residing in the perivascular niche in Ccr2-/- mice, their pro-angiogenic activity was reduced by the downregulation of Vegf. In conclusion, lacking CCR2 solely on tumor microenvironmental cells leads to enhanced tumor progression, whereby high numbers of TAMs infiltrate gliomas independently of the CCR2/CCL2 signal.
Keywords: CCR2/CCL2 signaling; GBM; tumor angiogenesis; tumor-associated macrophages (TAMs), blood vessel integrity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

