Current and Future Role of Tyrosine Kinases Inhibition in Thyroid Cancer: From Biology to Therapy
- PMID: 32668761
- PMCID: PMC7403957
- DOI: 10.3390/ijms21144951
Current and Future Role of Tyrosine Kinases Inhibition in Thyroid Cancer: From Biology to Therapy
Abstract
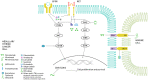
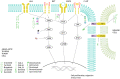
Thyroid cancer represents a heterogenous disease whose incidence has increased in the last decades. Although three main different subtypes have been described, molecular characterization is progressively being included in the diagnostic and therapeutic algorithm of these patients. In fact, thyroid cancer is a landmark in the oncological approach to solid tumors as it harbors key genetic alterations driving tumor progression that have been demonstrated to be potential actionable targets. Within this promising and rapid changing scenario, current efforts are directed to improve tumor characterization for an accurate guidance in the therapeutic management. In this sense, it is strongly recommended to perform tissue genotyping to patients that are going to be considered for systemic therapy in order to select the adequate treatment, according to recent clinical trials data. Overall, the aim of this article is to provide a comprehensive review on the molecular biology of thyroid cancer focusing on the key role of tyrosine kinases. Additionally, from a clinical point of view, we provide a thorough perspective, current and future, in the treatment landscape of this tumor.
Keywords: NTRK; RET; immunotherapy; thyroid cancer; tyrosine kinase inhibitors.
Conflict of interest statement
Teresa Alonso-Gordoa: Pfizer, Ipsen, Bristol-Myers Squibb, Roche, Eusa Pharma, MerckSharp&Dohme (C/A, SAB), Roche (RF). Enrique Grande: Pfizer, Bristol-Myers Squibb, Ipsen, Roche, Eisai, Eusa Pharma, MerckSharp&Dohme, Sanofi-Genzyme, Adacap, Novartis, PierreFabre, Lexicon, Celgene (C/A, SAB), Pfizer, AstraZeneca, MTEM/Threshold, Roche, Ipsen, Lexicon (RF). The rest of authors declares no conflict of interest. (C/A): Consulting/Advisory relationship; (RF) Research Funding; (SAB) Scientific Advisory Board.
Figures
References
-
- Howlader N., Noone A.M., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., et al. SEER Cancer Statistics Review, 1975–2016. National Cancer Institute; Bethesda, MD, USA: 2019.
-
- Liu J.W., Chen C., Loh E.W., Chu C.C., Wang M.Y., Ouyang H.J., Chang Y.T., Zhuang W.Z., Chou C.W., Huang D.J., et al. Tyrosine Kinase Inhibitors for Advanced or Metastatic Thyroid Cancer: A Meta-Analysis of Randomized Controlled Trials. Curr. Med. Res. Opin. 2018;34:795–803. doi: 10.1080/03007995.2017.1368466. - DOI - PubMed
-
- Lloyd R.V., Osamura Y.R., Kloppel G., Rosai J. WHO Classification of Tumours of Endocrine Organs. WHO Press; Geneva, Switzerland: 2017.
-
- Kimura E.T., Nikiforova M.N., Zhu Z., Knauf J.A., Nikiforov Y.E., Fagin J.A. High Prevalence of BRAF Mutations in Thyroid Cancer: Genetic Evidence for Constitutive Activation of the RET/PTC-RAS-BRAF Signaling Pathway in Papillary Thyroid Carcinoma. Cancer Res. 2003;63:1454–1457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

