Differential contribution of excitatory and inhibitory neurons in shaping neurovascular coupling in different epileptic neural states
- PMID: 32669018
- PMCID: PMC8054729
- DOI: 10.1177/0271678X20934071
Differential contribution of excitatory and inhibitory neurons in shaping neurovascular coupling in different epileptic neural states
Abstract
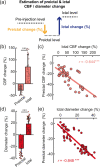
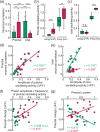
Understanding the neurovascular coupling (NVC) underlying hemodynamic changes in epilepsy is crucial to properly interpreting functional brain imaging signals associated with epileptic events. However, how excitatory and inhibitory neurons affect vascular responses in different epileptic states remains unknown. We conducted real-time in vivo measurements of cerebral blood flow (CBF), vessel diameter, and excitatory and inhibitory neuronal calcium signals during recurrent focal seizures. During preictal states, decreases in CBF and arteriole diameter were closely related to decreased γ-band local field potential (LFP) power, which was linked to relatively elevated excitatory and reduced inhibitory neuronal activity levels. Notably, this preictal condition was followed by a strengthened ictal event. In particular, the preictal inhibitory activity level was positively correlated with coherent oscillating activity specific to inhibitory neurons. In contrast, ictal states were characterized by elevated synchrony in excitatory neurons. Given these findings, we suggest that excitatory and inhibitory neurons differentially contribute to shaping the ictal and preictal neural states, respectively. Moreover, the preictal vascular activity, alongside with the γ-band, may reflect the relative levels of excitatory and inhibitory neuronal activity, and upcoming ictal activity. Our findings provide useful insights into how perfusion signals of different epileptic states are related in terms of NVC.
Keywords: Epilepsy; calcium imaging; cerebral blood flow; excitatory neuron; in vivo two-photon imaging; inhibitory neuron; neurovascular coupling.
Conflict of interest statement
Figures




References
-
- Oishi M, Ishida G, Morii K, et al. Ictal focal hyperperfusion demonstrated by arterial spin-labeling perfusion MRI in partial epilepsy status. Neuroradiology 2012; 54: 653–656. - PubMed
-
- Toledo M, Munuera J, Salas-Puig X, et al. Localisation value of ictal arterial spin-labelled sequences in partial seizures. Epileptic Disord 2011; 13: 336–339. - PubMed
-
- Pizzini F, Farace P, Zanoni T, et al. Pulsed-arterial-spin-labeling perfusion 3T MRI following single seizure: a first case report study. Epilepsy Res 2008; 81: 225–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

