Interleukin 6 reduces allopregnanolone synthesis in the brain and contributes to age-related cognitive decline in mice
- PMID: 32669383
- PMCID: PMC7529050
- DOI: 10.1194/jlr.RA119000479
Interleukin 6 reduces allopregnanolone synthesis in the brain and contributes to age-related cognitive decline in mice
Abstract
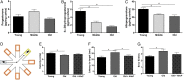
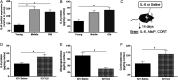
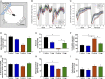
Cognitive decline with age is a harmful process that can reduce quality of life. Multiple factors have been established to contribute to cognitive decline, but the overall etiology remains unknown. Here, we hypothesized that cognitive dysfunction is mediated, in part, by increased levels of inflammatory cytokines that alter allopregnanolone (AlloP) levels, an important neurosteroid in the brain. We assessed the levels and regulation of AlloP and the effects of AlloP supplementation on cognitive function in 4-month-old and 24-month-old male C57BL/6 mice. With age, the expression of enzymes involved in the AlloP synthetic pathway was decreased and corticosterone (CORT) synthesis increased. Supplementation of AlloP improved cognitive function. Interestingly, interleukin 6 (IL-6) infusion in young animals significantly reduced the production of AlloP compared with controls. It is notable that inhibition of IL-6 with its natural inhibitor, soluble membrane glycoprotein 130, significantly improved spatial memory in aged mice. These findings were supported by in vitro experiments in primary murine astrocyte cultures, indicating that IL-6 decreases production of AlloP and increases CORT levels. Our results indicate that age-related increases in IL-6 levels reduce progesterone substrate availability, resulting in a decline in AlloP levels and an increase in CORT. Furthermore, our results indicate that AlloP is a critical link between inflammatory cytokines and the age-related decline in cognitive function.
Keywords: Alzheimer’s disease; aging; cognitive function; enzyme regulation; inflammation; neurosteroid; progesterone; steroid hormones.
Copyright © 2020 Parks et al. Published by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Cansino S., Torres-Trejo F., Estrada-Manilla C., Martinez-Galindo J. G., Hernandez-Ramos E., Ayala-Hernandez M., Gomez-Fernandez T., Ramirez-Gonzalez M. D., and Ruiz-Velasco S.. 2018. Factors that positively or negatively mediate the effects of age on working memory across the adult life span. Geroscience. 40: 293–303. - PMC - PubMed
-
- Godbout J. P., and Johnson R. W.. 2009. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunol. Allergy Clin. North Am. 29: 321–337. - PubMed
-
- Di Benedetto S., Muller L., Wenger E., Duzel S., and Pawelec G.. 2017. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci. Biobehav. Rev. 75: 114–128. - PubMed
-
- Compagnone N. A., and Mellon S. H.. 2000. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front. Neuroendocrinol. 21: 1–56. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

