Dispersal of Epithelium-Associated Pseudomonas aeruginosa Biofilms
- PMID: 32669459
- PMCID: PMC7364222
- DOI: 10.1128/mSphere.00630-20
Dispersal of Epithelium-Associated Pseudomonas aeruginosa Biofilms
Abstract
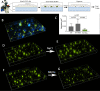
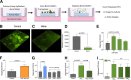
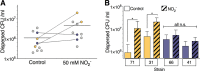
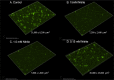
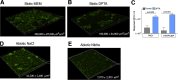
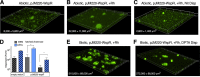
Pseudomonas aeruginosa grows in highly antibiotic-tolerant biofilms during chronic airway infections. Dispersal of bacteria from biofilms may restore antibiotic susceptibility or improve host clearance. We describe models to study biofilm dispersal in the nutritionally complex environment of the human airway. P. aeruginosa was cocultured in the apical surface of airway epithelial cells (AECs) in a perfusion chamber. Dispersal, triggered by sodium nitrite, a nitric oxide (NO) donor, was tracked by live cell microscopy. Next, a static model was developed in which biofilms were grown on polarized AECs without flow. We observed that NO-triggered biofilm dispersal was an energy-dependent process. From the existing literature, NO-mediated biofilm dispersal is regulated by DipA, NbdA, RbdA, and MucR. Interestingly, altered signaling pathways appear to be used in this model, as deletion of these genes failed to block NO-induced biofilm dispersal. Similar results were observed using biofilms grown in an abiotic model on glass with iron-supplemented cell culture medium. In cystic fibrosis, airway mucus contributes to the growth environment, and a wide range of bacterial phenotypes are observed; therefore, we tested biofilm dispersal in a panel of late cystic fibrosis clinical isolates cocultured in the mucus overlying primary human AECs. Finally, we examined dispersal in combination with the clinically used antibiotics ciprofloxacin, aztreonam and tobramycin. In summary, we have validated models to study biofilm dispersal in environments that recapitulate key features of the airway and identified combinations of currently used antibiotics that may enhance the therapeutic effect of biofilm dispersal.IMPORTANCE During chronic lung infections, Pseudomonas aeruginosa grows in highly antibiotic-tolerant communities called biofilms that are difficult for the host to clear. We have developed models for studying P. aeruginosa biofilm dispersal in environments that replicate key features of the airway. We found that mechanisms of biofilm dispersal in these models may employ alternative or additional signaling mechanisms, highlighting the importance of the growth environment in dispersal events. We have adapted the models to accommodate apical fluid flow, bacterial clinical isolates, antibiotics, and primary human airway epithelial cells, all of which are relevant to understanding bacterial behaviors in the context of human disease. We also examined dispersal agents in combination with commonly used antipseudomonal antibiotics and saw improved clearance when nitrite was combined with the antibiotic aztreonam.
Keywords: Pseudomonas aeruginosa; biofilm; cyclic-di-GMP; cystic fibrosis; dispersal; dispersion.
Copyright © 2020 Zemke et al.
Figures


References
-
- Cystic Fibrosis Foundation. 2018. Patient registry: annual data report 2018. https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2018-....
-
- DePas WH, Starwalt-Lee R, Van Sambeek L, Ravindra Kumar S, Gradinaru V, Newman DK. 2016. Exposing the three-dimensional biogeography and metabolic states of pathogens in cystic fibrosis sputum via hydrogel embedding, clearing, and rRNA labeling. mBio 7:e00796-16. doi:10.1128/mBio.00796-16. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
