Blood pressure-lowering effects of a Bowman-Birk inhibitor and its derived peptides in normotensive and hypertensive rats
- PMID: 32669617
- PMCID: PMC7363796
- DOI: 10.1038/s41598-020-66624-3
Blood pressure-lowering effects of a Bowman-Birk inhibitor and its derived peptides in normotensive and hypertensive rats
Abstract
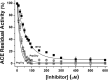
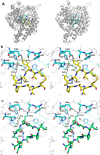
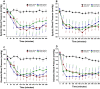
Bioactive plant peptides have received considerable interest as potential antihypertensive agents with potentially fewer side effects than antihypertensive drugs. Here, the blood pressure-lowering effects of the Bowman-Birk protease inhibitor, BTCI, and its derived peptides, PepChy and PepTry, were investigated using normotensive (Wistar-WR) and spontaneously hypertensive rats (SHR). BTCI inhibited the proteases trypsin and chymotrypsin, respectively, at 6 µM and 40 µM, a 10-fold greater inhibition than observed with PepTry (60 µM) and PepChy (400 µM). These molecules also inhibited angiotensin converting enzyme (ACE) with IC50 values of 54.6 ± 2.9; 24.7 ± 1.1; and 24.4 ± 1.1 µM, respectively, occluding its catalytic site, as indicated by molecular docking simulation, mainly for PepChy and PepTry. Gavage administration of BTCI and the peptides promoted a decrease of systolic and diastolic blood pressure and an increase of renal and aortic vascular conductance. These effects were more expressive in SHR than in WR. Additionally, BTCI, PepChy and PepTry promoted coronary vasodilation and negative inotropic effects in isolated perfused hearts. The nitric oxide synthase inhibitor blunted the BTCI and PepChy, with no cardiac effects on PepTry. The findings of this study indicate a therapeutic potential of BTCI and its related peptides in the treatment of hypertension.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Liu, X. Z. et al. Association between cardiovascular risk factors and stage 1 hypertension defined by the 2017 ACC/AHA guidelines. Clinical and experimental hypertension (New York, N.Y.: 1993), 1–7, 10.1080/10641963.2020.1714639 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

