Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target
- PMID: 32669658
- PMCID: PMC7362612
- DOI: 10.1038/s41573-020-0071-y
Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target
Abstract
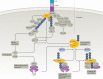
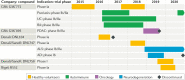
Receptor-interacting serine/threonine-protein kinase 1 (RIPK1) is a key mediator of cell death and inflammation. The unique hydrophobic pocket in the allosteric regulatory domain of RIPK1 has enabled the development of highly selective small-molecule inhibitors of its kinase activity, which have demonstrated safety in preclinical models and clinical trials. Potential applications of these RIPK1 inhibitors for the treatment of monogenic and polygenic autoimmune, inflammatory, neurodegenerative, ischaemic and acute conditions, such as sepsis, are emerging. This article reviews RIPK1 biology and disease-associated mutations in RIPK1 signalling pathways, highlighting clinical trials of RIPK1 inhibitors and potential strategies to mitigate development challenges.
Conflict of interest statement
J.Y. is a consultant for Denali Therapeutics and Sanofi. D.O. is an employee of Sanofi.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

