The pharmaceutical solvent N-methyl-2-pyrollidone (NMP) attenuates inflammation through Krüppel-like factor 2 activation to reduce atherogenesis
- PMID: 32669659
- PMCID: PMC7363918
- DOI: 10.1038/s41598-020-68350-2
The pharmaceutical solvent N-methyl-2-pyrollidone (NMP) attenuates inflammation through Krüppel-like factor 2 activation to reduce atherogenesis
Abstract
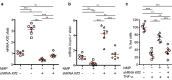
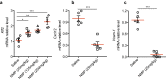
N-methyl-2-pyrrolidone (NMP) is a versatile water-miscible polar aprotic solvent. It is used as a drug solubilizer and penetration enhancer in human and animal, yet its bioactivity properties remain elusive. Here, we report that NMP is a bioactive anti-inflammatory compound well tolerated in vivo, that shows efficacy in reducing disease in a mouse model of atherosclerosis. Mechanistically, NMP increases the expression of the transcription factor Kruppel-like factor 2 (KLF2). Monocytes and endothelial cells treated with NMP express increased levels of KLF2, produce less pro-inflammatory cytokines and adhesion molecules. We found that NMP attenuates monocyte adhesion to endothelial cells inflamed with tumor necrosis factor alpha (TNF-α) by reducing expression of adhesion molecules. We further show using KLF2 shRNA that the inhibitory effect of NMP on endothelial inflammation and subsequent monocyte adhesion is KLF2 dependent. Enhancing KLF2 expression and activity improves endothelial function, controls multiple genes critical for inflammation, and prevents atherosclerosis. Our findings demonstrate a consistent effect of NMP upon KLF2 activation and inflammation, biological processes central to atherogenesis. Our data suggest that inclusion of bioactive solvent NMP in pharmaceutical compositions to treat inflammatory disorders might be beneficial and safe, in particular to treat diseases of the vascular system, such as atherosclerosis.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

