Nucleolar RNA polymerase II drives ribosome biogenesis
- PMID: 32669707
- PMCID: PMC7486236
- DOI: 10.1038/s41586-020-2497-0
Nucleolar RNA polymerase II drives ribosome biogenesis
Abstract
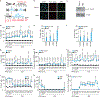
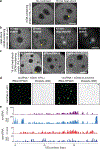
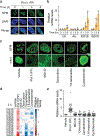
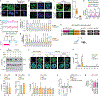
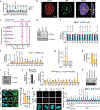
Proteins are manufactured by ribosomes-macromolecular complexes of protein and RNA molecules that are assembled within major nuclear compartments called nucleoli1,2. Existing models suggest that RNA polymerases I and III (Pol I and Pol III) are the only enzymes that directly mediate the expression of the ribosomal RNA (rRNA) components of ribosomes. Here we show, however, that RNA polymerase II (Pol II) inside human nucleoli operates near genes encoding rRNAs to drive their expression. Pol II, assisted by the neurodegeneration-associated enzyme senataxin, generates a shield comprising triplex nucleic acid structures known as R-loops at intergenic spacers flanking nucleolar rRNA genes. The shield prevents Pol I from producing sense intergenic noncoding RNAs (sincRNAs) that can disrupt nucleolar organization and rRNA expression. These disruptive sincRNAs can be unleashed by Pol II inhibition, senataxin loss, Ewing sarcoma or locus-associated R-loop repression through an experimental system involving the proteins RNaseH1, eGFP and dCas9 (which we refer to as 'red laser'). We reveal a nucleolar Pol-II-dependent mechanism that drives ribosome biogenesis, identify disease-associated disruption of nucleoli by noncoding RNAs, and establish locus-targeted R-loop modulation. Our findings revise theories of labour division between the major RNA polymerases, and identify nucleolar Pol II as a major factor in protein synthesis and nuclear organization, with potential implications for health and disease.
Conflict of interest statement
Figures






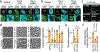


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

