A Clinical Program to Implement Repetitive Transcranial Magnetic Stimulation for Depression in the Department of Veterans Affairs
- PMID: 32669780
- PMCID: PMC7357884
A Clinical Program to Implement Repetitive Transcranial Magnetic Stimulation for Depression in the Department of Veterans Affairs
Abstract
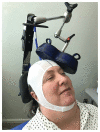
Background: Repetitive transcranial magnetic stimulation (rTMS) uses a device to create magnetic fields that cause electrical current to flow into targeted neurons in the brain. The most common clinical use of rTMS is for the treatment of major depressive disorder (MDD). The annual suicide rate of veterans has been higher than the national average; treating depression with rTMS would likely decrease suicide risk. MDD in many patients can be chronic and reoccurring with medication and psychotherapy providing inadequate relief.
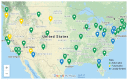
Methods: A pilot program was created to supply rTMS devices to 35 different sites in the VA nationwide in order to treat treatment-resistant depression.
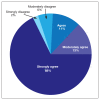
Conclusions: At time of analysis more than 950 veterans have started the program and 412 have finished. Nationwide, we have seen the depression scores decline, indicating an improvement in well-being. In addition, there is high patient satisfaction. Collecting data on a national level is a powerful way to examine rTMS efficacy and predictors of response which might be lost on a smaller subset of cases.
Copyright © 2020 Frontline Medical Communications Inc., Parsippany, NJ, USA.
Conflict of interest statement
Author disclosures The authors report no actual or potential conflicts of interest with regard to this article.
Figures
References
-
- Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522–534. doi: 10.1038/npp.2008.118. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources