Critical analysis of the use of β-site amyloid precursor protein-cleaving enzyme 1 inhibitors in the treatment of Alzheimer's disease
- PMID: 32669897
- PMCID: PMC7337240
- DOI: 10.2147/DNND.S41056
Critical analysis of the use of β-site amyloid precursor protein-cleaving enzyme 1 inhibitors in the treatment of Alzheimer's disease
Abstract
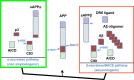
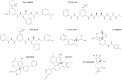
Alzheimer's disease (AD) is the major cause of dementia in the elderly and an unmet clinical challenge. A variety of therapies that are currently under development are directed to the amyloid cascade. Indeed, the accumulation and toxicity of amyloid-β (Aβ) is believed to play a central role in the etiology of the disease, and thus rational interventions are aimed at reducing the levels of Aβ in the brain. Targeting β-site amyloid precursor protein-cleaving enzyme (BACE)-1 represents an attractive strategy, as this enzyme catalyzes the initial and rate-limiting step in Aβ production. Observation of increased levels of BACE1 and enzymatic activity in the brain, cerebrospinal fluid, and platelets of patients with AD and mild cognitive impairment supports the potential benefits of BACE1 inhibition. Numerous potent inhibitors have been generated, and many of these have been proved to lower Aβ levels in the brain of animal models. Over 10 years of intensive research on BACE1 inhibitors has now culminated in advancing half a dozen of these drugs into human trials, yet translating the in vitro and cellular efficacy of BACE1 inhibitors into preclinical and clinical trials represents a challenge. This review addresses the promises and also the potential problems associated with BACE1 inhibitors for AD therapy, as the complex biological function of BACE1 in the brain is becoming unraveled.
Keywords: amyloid; aspartyl protease; dementia; neuregulin; secretase.
© 2014 Evin and Barakat.
Conflict of interest statement
The authors have no conflicts of interest in this work.
Figures

Similar articles
-
Highlights in BACE1 Inhibitors for Alzheimer's Disease Treatment.Front Chem. 2018 May 24;6:178. doi: 10.3389/fchem.2018.00178. eCollection 2018. Front Chem. 2018. PMID: 29881722 Free PMC article. Review.
-
Stepping closer to treating Alzheimer's disease patients with BACE1 inhibitor drugs.Transl Neurodegener. 2016 Jul 14;5:13. doi: 10.1186/s40035-016-0061-5. eCollection 2016. Transl Neurodegener. 2016. PMID: 27418961 Free PMC article. Review.
-
Investigational BACE inhibitors for the treatment of Alzheimer's disease.Expert Opin Investig Drugs. 2019 Nov;28(11):967-975. doi: 10.1080/13543784.2019.1683160. Epub 2019 Oct 29. Expert Opin Investig Drugs. 2019. PMID: 31661331 Review.
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
-
Role of BACE1 in Alzheimer's synaptic function.Transl Neurodegener. 2017 Aug 30;6:23. doi: 10.1186/s40035-017-0093-5. eCollection 2017. Transl Neurodegener. 2017. PMID: 28855981 Free PMC article. Review.
Cited by
-
Functional Foods: An Approach to Modulate Molecular Mechanisms of Alzheimer's Disease.Cells. 2020 Oct 23;9(11):2347. doi: 10.3390/cells9112347. Cells. 2020. PMID: 33114170 Free PMC article. Review.
-
Cooperation between neurovascular dysfunction and Aβ in Alzheimer's disease.Front Mol Neurosci. 2023 Aug 16;16:1227493. doi: 10.3389/fnmol.2023.1227493. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37654789 Free PMC article. Review.
-
The Anti-Aggregative Peptide KLVFF Mimics Aβ1-40 in the Modulation of Nicotinic Receptors: Implications for Peptide-Based Therapy.Biomedicines. 2022 Sep 8;10(9):2231. doi: 10.3390/biomedicines10092231. Biomedicines. 2022. PMID: 36140331 Free PMC article.
References
-
- Masters C, Beyreuther K. Alzheimer’s centennial legacy: prospects for rational therapeutic intervention targeting the Abeta amyloid pathway. Brain. 2006;129(Pt 11):2823–2839. - PubMed
-
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885–890. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

