Inhibition of Plasminogen Activator Inhibitor-1 Activation Suppresses High Fat Diet-Induced Weight Gain via Alleviation of Hypothalamic Leptin Resistance
- PMID: 32670063
- PMCID: PMC7327106
- DOI: 10.3389/fphar.2020.00943
Inhibition of Plasminogen Activator Inhibitor-1 Activation Suppresses High Fat Diet-Induced Weight Gain via Alleviation of Hypothalamic Leptin Resistance
Abstract
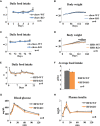
Leptin resistance is an important mechanism underlying the development and maintenance of obesity and is thus regarded as a promising target of obesity treatment. Plasminogen activator inhibitor 1 (PAI-1), a physiological inhibitor of tissue-type and urokinase-type plasminogen activators, is produced at high levels in adipose tissue, especially in states of obesity, and is considered to primarily be involved in thrombosis. PAI-1 may also have roles in inter-organ tissue communications regulating body weight, because PAI-1 knockout mice reportedly exhibit resistance to high fat diet (HFD)-induced obesity. However, the role of PAI-1 in body weight regulation and the underlying mechanisms have not been fully elucidated. We herein studied how PAI-1 affects systemic energy metabolism. We examined body weight and food intake of PAI-1 knockout mice fed normal chow or HFD. We also examined the effects of pharmacological inhibition of PAI-1 activity by a small molecular weight compound, TM5441, on body weight, leptin sensitivities, and expressions of thermogenesis-related genes in brown adipose tissue (BAT) of HFD-fed wild type (WT) mice. Neither body weight gain nor food intake was reduced in PAI-1 KO mice under chow fed conditions. On the other hand, under HFD feeding conditions, food intake was decreased in PAI-1 KO as compared with WT mice (HFD-WT mice 3.98 ± 0.08 g/day vs HFD-KO mice 3.73 ± 0.07 g/day, P = 0.021), leading to an eventual significant suppression of weight gain (HFD-WT mice 40.3 ± 1.68 g vs HFD-KO mice 34.6 ± 1.84 g, P = 0.039). Additionally, TM5441 treatment of WT mice pre-fed the HFD resulted in a marked suppression of body weight gain in a PAI-1-dependent manner (HFD-WT-Control mice 37.6 ± 1.07 g vs HFD-WT-TM5441 mice 33.8 ± 0.97 g, P = 0.017). TM5441 treatment alleviated HFD-induced systemic and hypothalamic leptin resistance, before suppression of weight gain was evident. Moreover, improved leptin sensitivity in response to TM5441 treatment was accompanied by increased expressions of thermogenesis-related genes such as uncoupling protein 1 in BAT (HFD-WT-Control mice 1.00 ± 0.07 vs HFD-WT-TM5441 mice 1.32 ± 0.05, P = 0.002). These results suggest that PAI-1 plays a causative role in body weight gain under HFD-fed conditions by inducing hypothalamic leptin resistance. Furthermore, they indicate that pharmacological inhibition of PAI-1 activity is a potential strategy for alleviating diet-induced leptin resistance in obese subjects.
Keywords: arcuate nucleus; food intake; leptin resistance; obesity; plasminogen activator inhibitor-1; thermogenesis.
Copyright © 2020 Hosaka, Yamada, Takahashi, Dan, Kaneko, Kodama, Asai, Munakata, Endo, Sugawara, Kawana, Yamamoto, Izumi, Sawada, Imai, Miyata and Katagiri.
Figures



References
-
- Asai Y., Yamada T., Tsukita S., Takahashi K., Maekawa M., Honma M., et al. (2017). Activation of the Hypoxia Inducible Factor 1alpha Subunit Pathway in Steatotic Liver Contributes to Formation of Cholesterol Gallstones. Gastroenterology 152 (6), 1521–1535 e1528. 10.1053/j.gastro.2017.01.001 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

