The Contribution of Surface Tension-Dependent Alveolar Septal Stress Concentrations to Ventilation-Induced Lung Injury in the Acute Respiratory Distress Syndrome
- PMID: 32670073
- PMCID: PMC7332732
- DOI: 10.3389/fphys.2020.00388
The Contribution of Surface Tension-Dependent Alveolar Septal Stress Concentrations to Ventilation-Induced Lung Injury in the Acute Respiratory Distress Syndrome
Abstract
In the acute respiratory distress syndrome (ARDS), surface tension, T, is likely elevated. And mechanical ventilation of ARDS patients causes ventilation-induced lung injury (VILI), which is believed to be proportional to T. However, the mechanisms through which elevated T may contribute to VILI have been under-studied. This conceptual analysis considers experimental and theoretical evidence for static and dynamic mechanical mechanisms, at the alveolar scale, through which elevated T exacerbates VILI; potential causes of elevated T in ARDS; and T-dependent means of reducing VILI. In the last section, possible means of reducing T and improving the efficacy of recruitment maneuvers during mechanical ventilation of ARDS patients are discussed.
Keywords: acute respiratory distress syndrome (ARDS); mechanical ventilation; recruitment maneuvers; stress concentrations; surface tension; ventilation-induced lung injury (VILI).
Copyright © 2020 Perlman.
Figures
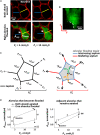
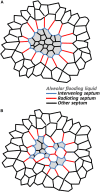

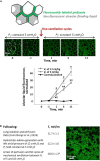
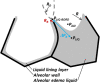
References
-
- Anzueto A., Baughman R. P., Guntupalli K. K., Weg J. G., Wiedemann H. P., Raventós A. A., et al. (1996). Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. Exosurf acute respiratory distress syndrome sepsis study group. N. Engl. J. Med. 334 1417–1421. 10.1056/NEJM199605303342201 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

