Molecular Phenotyping of White Striping and Wooden Breast Myopathies in Chicken
- PMID: 32670085
- PMCID: PMC7328665
- DOI: 10.3389/fphys.2020.00633
Molecular Phenotyping of White Striping and Wooden Breast Myopathies in Chicken
Abstract
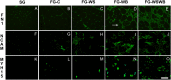
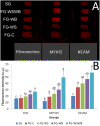
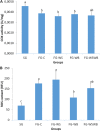
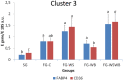
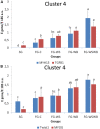
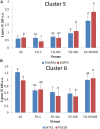
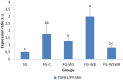
The White Striping (WS) and Wooden Breast (WB) defects are two myopathic syndromes whose occurrence has recently increased in modern fast-growing broilers. The impact of these defects on the quality of breast meat is very important, as they greatly affect its visual aspect, nutritional value, and processing yields. The research conducted to date has improved our knowledge of the biological processes involved in their occurrence, but no solution has been identified so far to significantly reduce their incidence without affecting growing performance of broilers. This study aims to follow the evolution of molecular phenotypes in relation to both fast-growing rate and the occurrence of defects in order to identify potential biomarkers for diagnostic purposes, but also to improve our understanding of physiological dysregulation involved in the occurrence of WS and WB. This has been achieved through enzymatic, histological, and transcriptional approaches by considering breast muscles from a slow- and a fast-growing line, affected or not by WS and WB. Fast-growing muscles produced more reactive oxygen species (ROS) than slow-growing ones, independently of WS and WB occurrence. Within fast-growing muscles, despite higher mitochondria density, muscles affected by WS or WB defects did not show higher cytochrome oxidase activity (COX) activity, suggesting altered mitochondrial function. Among the markers related to muscle remodeling and regeneration, immunohistochemical staining of FN1, NCAM, and MYH15 was higher in fast- compared to slow-growing muscles, and their amount also increased linearly with the presence and severity of WS and WB defects, making them potential biomarkers to assess accurately their presence and severity. Thanks to an innovative histological technique based on fluorescence intensity measurement, they can be rapidly quantified to estimate the injuries induced in case of WS and WB. The muscular expression of several other genes correlates also positively to the presence and severity of the defects like TGFB1 and CTGF, both involved in the development of connective tissue, or Twist1, known as an inhibitor of myogenesis. Finally, our results suggested that a balance between TGFB1 and PPARG would be essential for fibrosis or adiposis induction and therefore for determining WS and WB phenotypes.
Keywords: White Striping; Wooden Breast; mitochondria; molecular phenotype; muscle remodeling.
Copyright © 2020 Praud, Jimenez, Pampouille, Couroussé, Godet, Le Bihan-Duval and Berri.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

