Facilitated Subcutaneous Immunoglobulin Replacement Therapy in Clinical Practice: A Two Center, Long-Term Retrospective Observation in Adults With Primary Immunodeficiencies
- PMID: 32670265
- PMCID: PMC7326142
- DOI: 10.3389/fimmu.2020.00981
Facilitated Subcutaneous Immunoglobulin Replacement Therapy in Clinical Practice: A Two Center, Long-Term Retrospective Observation in Adults With Primary Immunodeficiencies
Abstract
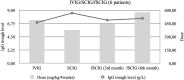
Facilitated subcutaneous immunoglobulin (fSCIG) replacement therapy is the latest method of IgG administration; however, real-life data are limited. We retrospectively analyzed the everyday experience of fSCIG administration, particularly, the method used to switch from intravenous immunoglobulin (IVIG) or subcutaneous immunoglobulin (SCIG) to fSCIG and the dosing modifications required. Of the 39 adult patients with primary immunodeficiency (PID) who received fSCIG, 34 remained on the therapy at the end of the study. The median observation time was 18 (range, 3-24) months. Two patients were IgG-treatment-naïve; 23 had previously received IVIG and 14 had received SCIG. In 25 cases, a non-ramp-up dosing mode was used to switch to fSCIG (including two half-monthly doses given biweekly in 14 cases, and full monthly doses given in 11 cases), a ramp-up mode was used in six cases; other methods were used in eight cases. The median IgG trough level at baseline was 7.9 g/L (n = 38), 7.9 g/L (n = 32) at Month 6, 9.0 g/L (n = 30) at Month 12, 8.6 g/L (n = 22) at Month 18, and 9.0 g/L (n = 11) at Month 24. No serious bacterial infections or hospitalizations due to PID complications occurred. At the end of the study, 24 patients (71%) received fSCIG every 4 weeks, six (18%) received fSCIG every 3 weeks, and four (12%) received fSCIG biweekly. In conclusion, our study provides real-life evidence of clinical efficacy of personalized fSCIG treatment when switching from prior immunoglobulin replacement using various switching modes and dosing frequencies.
Keywords: common variable immunodeficiency; fSCIG; hypogammaglobulinemia; personalized approach; pregnancy; primary immunodeficiency; real-life data; replacement immunoglobulin therapy.
Copyright © 2020 Wiesik-Szewczyk, Sołdacki, Paczek and Jahnz-Różyk.
Figures
Similar articles
-
Real-World Experiences With Facilitated Subcutaneous Immunoglobulin Substitution in Patients With Hypogammaglobulinemia, Using a Three-Step Ramp-Up Schedule.Front Immunol. 2021 Apr 27;12:670547. doi: 10.3389/fimmu.2021.670547. eCollection 2021. Front Immunol. 2021. PMID: 34012453 Free PMC article.
-
Facilitated Subcutaneous Immunoglobulin Treatment Increases the Quality of Life and Decreases the Number of Infections and Hospitalizations in Children with Primary Immunodeficiencies.Int Arch Allergy Immunol. 2024;185(4):382-391. doi: 10.1159/000534900. Epub 2024 Jan 19. Int Arch Allergy Immunol. 2024. PMID: 38246144
-
Effects of Body Mass and Age on the Pharmacokinetics of Subcutaneous or Hyaluronidase-facilitated Subcutaneous Immunoglobulin G in Primary Immunodeficiency Diseases.J Clin Immunol. 2023 Nov;43(8):2127-2135. doi: 10.1007/s10875-023-01572-x. Epub 2023 Sep 29. J Clin Immunol. 2023. PMID: 37773562 Free PMC article.
-
Nuts and Bolts of Subcutaneous Therapy.Immunol Allergy Clin North Am. 2020 Aug;40(3):527-537. doi: 10.1016/j.iac.2020.04.002. Epub 2020 Jun 9. Immunol Allergy Clin North Am. 2020. PMID: 32654697 Review.
-
Long-Term Experience of Subcutaneous Immunoglobulin Therapy in Pediatric Primary Immunodeficient Patients with Low and Normal Body Weight.J Clin Immunol. 2022 Jan;42(1):64-71. doi: 10.1007/s10875-021-01144-x. Epub 2021 Oct 7. J Clin Immunol. 2022. PMID: 34617265 Review.
Cited by
-
Insights into Patient Experiences with Facilitated Subcutaneous Immunoglobulin Therapy in Primary Immune Deficiency: A Prospective Observational Cohort.J Clin Immunol. 2024 Aug 5;44(8):169. doi: 10.1007/s10875-024-01771-0. J Clin Immunol. 2024. PMID: 39098942 Free PMC article.
-
Real-World Effectiveness, Safety, and Tolerability of Facilitated Subcutaneous Immunoglobulin 10% in Secondary Immunodeficiency Disease: A Systematic Literature Review.J Clin Med. 2025 Feb 12;14(4):1203. doi: 10.3390/jcm14041203. J Clin Med. 2025. PMID: 40004732 Free PMC article. Review.
-
Immunoglobulin replacement therapies in inborn errors of immunity: a review.Front Pediatr. 2024 Feb 15;12:1368755. doi: 10.3389/fped.2024.1368755. eCollection 2024. Front Pediatr. 2024. PMID: 38425666 Free PMC article. Review.
-
A clinician's guide for administration of high-concentration and facilitated subcutaneous immunoglobulin replacement therapy in patients with primary immunodeficiency diseases.Allergy Asthma Clin Immunol. 2022 Sep 30;18(1):87. doi: 10.1186/s13223-022-00726-7. Allergy Asthma Clin Immunol. 2022. PMID: 36180928 Free PMC article. Review.
-
Advances in CRISPR/Cas gene therapy for inborn errors of immunity.Front Immunol. 2023 Mar 27;14:1111777. doi: 10.3389/fimmu.2023.1111777. eCollection 2023. Front Immunol. 2023. PMID: 37051232 Free PMC article. Review.
References
-
- Wiesik-Szewczyk E, Zietkiewicz M, Matyja-Bednarczyk A, Napiórkowska-Baran K, Suchanek H, Jahnz-Rózyk K. The first Polish cohort of adult patients with common variable immunodeficiency from 4 specialized centers: do we provide standards of care? Pol Arch Intern Med. (2018) 128:563–6. 10.20452/pamw.4315 - DOI - PubMed
-
- Pulvirenti F, Cinetto F, Pecoraro A, Carrabba M, Crescenzi L, Neri R, et al. . Health-related quality of life in patients with CVID under different schedules of immunoglobulin administration: prospective multicenter study. J Clin Immunol. (2019) 39:159–70. 10.1007/s10875-019-0592-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

